A 72-year-old man with a 40 pack-year smoking history is diagnosed with advanced lung cancer. His past medical history includes only hypertension. His creatinine level is 1.5 mg/dL. He lives with his daughter and is employed part-time as a volunteer in a gift shop. His daughter, a nurse, is concerned about the risks of myelosuppression with chemotherapy drugs and wonders about the benefit of chemotherapy at his age.
A 65-year-old woman with a diagnosis of node-positive breast cancer has been recommended to receive chemotherapy after surgery. She also has a history of diabetes, controlled with drugs, and she still maintains an active lifestyle. She wants to be treated with a regimen that offers her the maximum benefit, but is concerned that her age may increase the risk of infections and fatigue from the drugs.
Their primary physician decides to review the myelosuppressive toxicity of chemotherapy drugs in the elderly to help these patients make an informed decision.
Physiology of Aging as it Relates to Bone Marrow Function and Reserve
Aging is a universal phenomenon that affects all normal cells, tissues, organ systems, and organisms. Accordingly, the bone marrow undergoes changes with age. Age-related hematologic changes are reflected by a decline in bone marrow cellularity, an increased risk of myeloproliferative diseases and anemia, and a declining adaptive immunity.
The percentage of marrow space occupied by the hematopoietic tissue declines from 90% to 50% over the first 30 years of life and levels off thereafter, followed by a second decline to 30% at age 70, with the remaining space being taken up by fat. A similar change occurs in the thymus, where involution begins at an earlier age and is reflected anatomically by a reduction in lymphoid mass with an increase in fat, and functionally by a steady decrease in the production of naive T cells. Thus, fat infiltration into the bone marrow and thymus is associated with a reduced capacity to make new blood cells and diminished adaptive immune responses in late life.
Although age-related change in the bone marrow is well described, the exact mechanisms that regulate these changes remain speculative. For example, it remains unclear whether the age-associated expansion of marrow fat is a cause or an effect of aging and whether the changes seen in bone marrow and thymus are intrinsically related. All blood cells are derived from marrow pluripotent stem cells, which comprise 10% of the cellular fraction of cord blood but less than 1% of all adult bone marrow. Hematopoietic stem cells have a unique ability to self-renew, proliferate, and differentiate into every lineage of mature blood cells. Hematopoietic stem cells then give rise to two distinct multipotent stem cells within the bone marrow. Myeloid stem cells are precursors of granulocytes, monocytes, erythrocytes, and platelets; lymphoid stem cells are precursors of lymphocytes and plasma cells. There is always a large pool of maturing progenitors for each lineage within the bone marrow, allowing for rapid recruitment and release of cells in times of stress. The factors responsible for the constant turnover of these mature cells both inside the bone marrow and in the peripheral blood are poorly understood. However, there is evidence to support a role for both cell-intrinsic genetic programs and several hematopoietic growth factors within the bone marrow microenvironment in the regulation of hematopoiesis. Although a number of measureable changes occur in the stem cell compartment with aging, these changes do not compromise hematopoiesis in the absence of disease. Even when bone marrow is donated from a 65-year-old person to an HLA-matched younger recipient, the transferred marrow supports hematopoiesis for the life of the recipient.
Unlike the commonly held notion that stem cell compartments diminish either in number or function with age, ultimately resulting in an inability to meet homeostatic demands, age-related hematopoietic stem cell (HSC) changes appear to be an exception, at least for murine species in which this question has been most directly addressed. Early work demonstrated that marrow serially-transplanted could reconstitute hematopoietic function for an estimated 15 to 20 life spans. Furthermore, the capacity for old marrow to reconstitute proved superior to that of young marrow. Subsequently, a number of investigators using a variety of techniques have concluded that HSC concentration in old mice is approximately twice that found in the young. Some evidence suggests that the intrinsic function of HSCs changes somewhat with age, most notably with a shift in lineage potential from lymphoid to myeloid development. This may contribute to an observed relative increase in neutrophils and decrease in lymphocytes in the peripheral blood of older people. Although no significant change is seen in the peripheral blood leukocyte count with aging, several qualitative neutrophil defects have been described. For example, a decreased respiratory burst response to soluble signals, defective phagocytosis, and impaired neutrophil migration to sites of stress have been described. Although the exact cause for these functional changes has not been clarified, it may be associated with an age-related alteration in actin cytoskeleton and receptor expression in leukocytes. There is a decrease in the peripheral lymphocyte count that is first noticeable in the fourth decade, with a gradual progression thereafter throughout the remainder of the life span. Studies have also demonstrated qualitative alterations in T-lymphocyte function in the elderly. Although the HSC compartment is sufficient to maintain normal blood counts in older individuals who are healthy, there is now a substantial literature indicating that bone marrow reserve is diminished in the older compared to younger cancer patient, and this becomes of clinical importance for patients receiving chemotherapy or radiation.
Clinical Observations: Myelosuppression in Older Cancer Patients
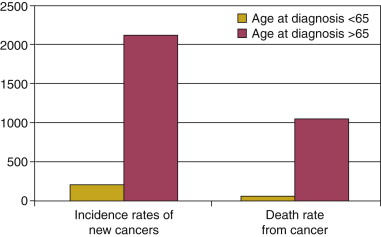
According to the 2002-2006 Surveillance, Epidemiology, and End Results (SEER) data from the National Cancer Institute, more than 50% of cancers are first diagnosed in patients older than 65 years. Furthermore, this group sustains approximately 70% of all cancer deaths ( Figure 13-1 ). Physicians tend to defer referring older patients for chemotherapy as compared to younger patients despite evidence showing that the majority of the elderly are willing to accept cytotoxic treatment for possible benefit. Elderly patients who are referred to treatment are also likely to receive attenuated treatment when compared to younger patients. In the Annual Report to the Nation on the Status of Cancer, 1975-2002, Featuring Population-Based Trends in Cancer Treatment published in the Journal of National Cancer Institute in 2005, evaluation of cancer care delivery consistently showed that the elderly were less likely to receive standard therapy despite adjusting for comorbidities. The perceived risk-benefit effect of chemotherapy, particularly concerns about increased myelosuppressive toxicity in the context of advanced age and declining health status, may influence this decision. Understanding the risk of hematological toxicity, the mechanisms related to its possible increased frequency in the elderly, and its best management may further improve treatment outcomes in the geriatric population.
Benefit to Older Patients from Chemotherapy
Several studies in different types of cancer including colon, lung, breast, and lymphoma have shown that older patients treated with standard intensive regimens derive similar benefit in terms of response. EORTC conducted a MEDLINE review of phase III and phase II studies of chemotherapy in the adjuvant and metastatic treatment setting of colon cancer. They recommended that cytotoxic combination chemotherapy regimens (5-FU with irinotecan or oxaliplatin) offered similar benefits in older patients and should be considered standard therapy for fit older patients. In lung cancer, both the European Organisation for Research and Treatment of Cancer (EORTC) Elderly Task Force and the International Society for Geriatric Oncology found elderly patients seem to derive the same benefit from adjuvant chemotherapy as younger patients. Elderly patients who receive standard-dose intensive treatments during treatment for non-Hodgkin lymphomas (NHL) and breast cancer also have comparable rates of response. Most of these studies also revealed that the chemotherapy tolerability is similar among the older and younger patients. In addition, despite a significant decrease in functional status during treatment, most elderly return to their pretreatment levels after completion of therapy. However, greater hematological toxicity is seen in most studies in the elderly compared to the younger patients.
One of the earliest papers published evaluating the effects of chemotherapy on the elderly was a review of 19 Eastern Cooperative Oncology Group studies of advanced cancer in eight disease sites. This study compared response rates and toxicity among 5459 patients younger than 70 to 780 patients older than 70. It reported that the elderly patients have similar response rates and survival expectancy as compared to the younger patients. Hematological toxicity was the most frequent side effect observed and was higher in those older than 70 years. Severe or worse toxicities (leukocytes less than 2000/mm , platelets less than 50,000/mm , neutrophils less than 1000/mm , or necessity for transfusions) was related to type of cancer, being more prevalent in patients with head and neck, ovarian, or gastric carcinomas; melanoma; and sarcoma. Since then, advanced age has been consistently shown to predict an increased incidence of neutropenia, anemia, and infectious complications in multivariate regression risk models in a number of tumor types. In non-Hodgkin lymphoma patients, a risk model incorporating increasing age (10-year increments), increased dose, prior chemotherapy, recent infection, and low baseline albumin (less than 35 g/L) predicted higher risk of first-cycle febrile neutropenia with a sensitivity of 81% and a specificity of 80%. Increasing age also was predictive for risk of febrile neutropenia in any cycle in this model ( Figure 13-2 ). Pharmacokinetic studies of drugs in the elderly also demonstrate increased risk for neutropenia despite a failure to demonstrate an age-associated change in clearance.

Myelosuppression in Elderly Lung Cancer Patients
The majority (more than 50%) of non-small cell lung cancer (NSCLC) patients are older than 65 years. Chemotherapy protocols including platinum, non–platinum-based treatments like gemcitabine and vinorelbine, and taxane-based chemotherapy are widely used in treatment in either the adjuvant or advanced setting. Several retrospective analyses have found that elderly lung cancer patients benefit equally from treatment in the adjuvant setting but at the expense of increased hematological toxicity. Toxicity analysis in pooled studies of cisplatin for adjuvant treatment of lung cancer identified grade 3 neutropenia in more than 50% of patients older than 65 years.
Anemia and neutropenia are the two most common short-term toxicities reported in elderly patients undergoing chemotherapy for advanced lung cancer, occurring in up to 20% of patients. A recent prospective study in stage III or IV lung cancer patients also demonstrated a higher incidence (8% versus 2%) of febrile neutropenia in elderly patients older than 75 years as compared to those younger than 55 years. Treatment of advanced lung cancer with combination chemotherapy regimens can also cause increased myelosuppression in the elderly. More than 80% of patients older than 65 years experienced grade 3/4 neutropenia in the TAX-326 study, which evaluated three platinum-based regimens with docetaxel/vinorelbine. In another study in which more than 15% of those enrolled were older than 70 years, cisplatin/paclitaxel or etoposide chemotherapy was associated with greater than grade 3 leukopenia in more than 70% of the elderly and anemia in more than 25%. Incidence of grade 3/4 thrombocytopenia is usually low in most studies, around 10%, but this is significantly increased in regimens incorporating gemcitabine and/or carboplatin, where rates as high as 35% have been reported in the elderly population. Combination regimens used in the treatment of small cell lung cancer also have a high incidence of anemia (28%), neutropenia (77%), and thrombocytopenia (26%) when evaluated in the elderly population.
Myelosuppression in Breast Cancer Patients
Older patients treated with chemotherapy for breast cancer have a higher risk of being hospitalized for hematological complications, including febrile neutropenia. Increased hematological toxicity has been observed in the metastatic setting in elderly breast cancer patients. The Piedmont Oncology Group published their experience of hematological toxicity in the elderly in five trials conducted between 1974 and 1989 and reported twice the rates of severe neutropenia for those older than 70 who were treated with cyclophosphamide/doxorubicin regimens for advanced breast cancer. In the adjuvant setting, combination regimens of cyclophosphamide and doxorubicin, with or without paclitaxel have high rates of grade 4 neutropenia, ranging from 8% to 42% in elderly patients. Fluorouracil-based combinations with methotrexate/doxorubicin and cyclophosphamide (CMF/CAF) appear to be associated with a slightly decreased risk of hematological toxicity in the elderly. For instance, in the International Breast Cancer Study Group Trial VII, which evaluated addition of CMF to tamoxifen in the adjuvant setting, grade 3 neutropenia (neutrophil count < 750/μL) was seen in only 2.6% of patients and thrombocytopenia (< 50,000/μL) in 4.0%. Very low rates of neutropenia and thrombocytopenia were also seen in the CALGB trial 8641, which tested different doses and durations of the CAF regimen.
Studies of toxicity in other solid tumors replicate findings in breast and lung cancer, with high rates of hematological toxicity in the elderly.
Myelosuppressive Toxicity in Malignant Lymphomas
Myelosuppressive toxicity is very common during treatment of hematological malignancies, as patients may start treatment with decreased values secondary to bone marrow invasion. In one study of 359 patients treated for malignant lymphoma ranging in age from 18 to 87 years and with 63% older than 50 years, more than 34% had hemoglobin levels less than 12 g/dL before starting chemotherapy, increasing to 49% during chemotherapy. Interestingly 53% of patients with grade 1 anemia by NCI criteria had anemia-related symptoms but were not offered any intervention. Incidence of grade 4 neutropenia ranged from 4% to 91% in an analysis of 11 trials of elderly patients treated for non-Hodgkin lymphomas. The trials differed in the type of regimens used and also in the schedules administered, which most likely explains this wide range in incidence. A subanalysis of a phase II trial in non-Hodgkin lymphoma in patients older than 60 years treated with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) therapy, reported severe neutropenia in 24% of cycles in patients aged 61 to 69 years and in 73% of cycles in patients aged 70 years or older. There was also a higher rate of neutropenic fever occurring in 8% of patients aged 61 to 69 years and in 42% of patients aged 70 years or older. Severe thrombocytopenia (<20,000/mL) was seen in 5% of patients aged 61 to 69 years and in 42% of patients aged 70 years or older.
It is clear from the these data that myelosuppressive toxicity is definitely increased in the elderly as compared to the younger population. However, these retrospective analyses are hampered by limited representation of the elderly population, and the use of different definitions to evaluate the “elderly.” It has been estimated that only 22% of patients in clinical trials are older than 65 years, and only about 10% are older than 70 years. Data on myelosuppressive effects of individual chemotherapy drugs in the elderly are very limited. Accordingly, we may be both overestimating efficacy and underestimating toxicity in the elderly on the basis of these trials.
Toxicities in clinical trials are generally assessed by WHO criteria, or more recently the NCI criteria, with grades from 1 through 4. However, these may be simplistic in their representation of the profound functional effects that can occur. For instance, by NCI Common Toxicity Criteria, grade 1 (mild) anemia represents a hemoglobin level of 10.0 g/dL to within normal limits; grade 2 (moderate), 8.0-10.0 g/dL; grade 3 (serious or severe), 6.5-7.9 g/dL; and grade 4 (life-threatening), less than 6.5 g/dL. Most studies report only grade 3 or 4 toxicities, as these represent the most severe toxicity. This likely underestimates the overall burden, as studies have shown that even a mild decrease in hemoglobin levels from normal in the elderly can be associated with increased morbidity and mortality. Thus a true estimate of impact of anemia in the elderly is lacking.
Consequences of Myelosuppressive Toxicity
One of the major consequences of increased hematological toxicity is that it increases the risk of suboptimal chemotherapy delivery in this group. Dose reductions are frequently employed upfront to reduce the risk of toxicity. Although this strategy has been successful in reducing myelosuppressive toxicity in the elderly population, it is clearly associated with inferior outcomes and is probably one of the major factors contributing to increased mortality from cancer among the elderly. Still, hospitalizations and mortality from febrile neutropenia are greater for elderly patients than for younger patients despite decreased dose intensity. Myelosuppressive toxicity may also be persistent and decrease quality of life even long after completion of chemotherapy. Analysis of the Medicare SEER database to evaluate the incidence of chemotherapy toxicity-related conditions for 14 chemotherapy agents in elderly patients with non-small cell cancer revealed that the incidence of anemia increased from 20% to 35.9% during chemotherapy, and further increased to 30.7% to 37.6% when evaluated 3 months after chemotherapy. In a multivariate analysis, carboplatin, cisplatin, vinorelbine, paclitaxel and gemcitabine were significantly associated with development of long-term neutropenia and thrombocytopenia.
The economic burden in terms of supportive care during inpatient and outpatient hospitalization for febrile neutropenia is substantial, particularly in the management of hematological malignancies. Neutropenia has also been shown to influence the incidence and duration of nonhematological toxicities and to substantially decrease quality of life. Worsening or new-onset anemia during the course of chemotherapy significantly correlates with decreased performance status, increased fatigue, and overall decreased quality of life. Anemia also correlates with decreased survival in patients being treated for lymphomas and solid tumors. Major bleeding episodes associated with thrombocytopenia can lead to treatment delays and hospitalization, with resultant morbidity.
Preventing and Managing Myelosuppression in the Older Cancer Patient
Clearly, the hematological toxicity and adverse consequences from the same are increased in the elderly. Attempts to reduce this side effect include identifying and modifying treatment-related and patient-related factors that contribute to this increase.
Modification of Chemotherapy to Reduce Toxicity
Reduction in dose intensity has long been adopted as a way of reducing myelosuppression in the elderly population. For instance, in a retrospective nationwide survey of 567 oncology practices involving 4,522 patients with aggressive NHL treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP); CHOP-rituximab (CHOP-R); or cyclophosphamide, mitoxantrone, vincristine, and prednisone (CNOP) elderly patients (older than 60 years) were more likely to receive less than 85% of the planned dose intensity, with an increased proportion of patients receiving this reduced dose for successive cycles. However, as mentioned earlier, any benefit from reduced toxicity is countered by the reduced survival outcomes observed with decreased dose intensity or dose reductions.
Some chemotherapy regimens or drugs may be more myelotoxic than others in the elderly population. In a retrospective analysis of 132 patients aged 65 years or older with primary invasive breast cancer who received one of three different chemotherapy protocols: cyclophosphamide, methotrexate, fluorouracil (CMF); doxorubicin and cyclophosphamide (AC); or AC plus paclitaxel or docetaxel (AT-T); patients who received AC-based regimens were more likely to experience grade 3 or 4 hematological toxicity (32% versus 18%) and/or grade 3 neutropenic infection (29% versus 2%) as compared to those on the CMF regimen. The type of chemotherapy regimen (anthracycline compared to CMF) was a better predictor for toxicity than increased age or comorbidity score. In the recent adjuvant CALGB trial (49907), breast cancer patients 65 years and older with a performance score of 0 to 2 were randomized to receive either “conventional therapy” (doxorubicin/cyclophosphamide or cyclophosphamide/methotrexate/fluorouracil [CMF]) or capecitabine. Approximately 50% of patients in the combination arm experienced severe hematological toxicity compared to less than 5% in the arm treated with capecitabine alone. However, response rates and survival were significantly better for those receiving combination therapies.
Similarly, in lung cancer patients, both docetaxel and vinorelbine demonstrated comparable efficacy in a phase III trial in older patients in terms of median survival but docetaxel was associated with more grade 3 to 4 neutropenia (82.9% vs. 69.2%). Thus in a patient in whom occurrence of neutropenia will be life-threatening, vinorelbine is a reasonable option.
Incidence of specific myelosuppressive toxicities may also differ among regimens. In the elder specific subanalysis of the TAX-326 trial, patients with IIIB-IV NSCLC were randomized to docetaxel and cisplatin, docetaxel and carboplatin, or vinorelbine and cisplatin. The incidence of grade 3-4 thrombocytopenia and neutropenia was much higher in the elderly population on the docetaxel/carboplatin arm as compared to those on the other two arms. Grade 3-4 anemia was higher in the vinorelbine arm, occurring in 25% of those older than 65 years, as compared to 13.3% in the docetaxel/carboplatin arm and 5.4% in the docetaxel and cisplatin arm.
Recently, a number of new drugs have been evaluated in the first-line and second-line treatment of lung cancer. Pemetrexed, liposomal doxorubicin, and the newer targeted agents like erlotinib or gefitinib may be less myelosuppressive and regimens incorporating these agents may be used more frequently to reduce the incidence of myelosuppression in the elderly. Interestingly, in a large analysis of advanced lung cancer patients older than 65 years, patients treated in combination with bevacizumab had a 60% rate of more than twofold increase in neutropenia within 2 months after chemotherapy. Another caveat with the use of targeted treatments in the elderly is that although hematological toxicity is reduced, nonhematological toxicity may be significantly enhanced, limiting the use of some of these drugs in this population.
Elderly patients with advanced lung and breast cancer also may be better served with a single chemotherapeutic agent than with combination regimens. A large randomized phase III trial (the Multicenter Italian Lung Cancer in the Elderly Study) of 700 elderly patients showed that the combination of vinorelbine plus gemcitabine was no more effective than single-agent vinorelbine or gemcitabine in the treatment of elderly patients with advanced NSCLC. Combination chemotherapy resulted in more thrombocytopenia (3%) than single-agent vinorelbine (<1%) and more neutropenia (13%), than single-agent gemcitabine (7%).
Management of cancer in the elderly requires a careful consideration of the ultimate goal of treatment (cure versus palliation) and appropriate use of regimens to avoid further harm in this subgroup of patients.
Elder-specific trials with a gentler treatment-based approach have been proposed to improve management of older cancer patients ( Table 13-1 ). A pooled analysis of toxicity and outcomes in 118 elderly patients treated in two elderly-specific (inclusion criteria ≥ 65 years) and two nonspecific trials was conducted by the North Central Cancer Treatment Group. Grade 3 or worse hematological toxicity was seen in 68% of the elderly in age-unspecified trials as compared to 10% in the elderly-specific trials (neutropenia in 56% and 9% of patients, and thrombocytopenia in 14% and 1% of patients, respectively). There were no statistically significant differences with regard to treatment efficacy. However, conclusions from these trials are limited because of the small number of participants in the elder-specific trials.
| Regimen | Anemia | Neutropenia | Thrombocytopenia | |||
|---|---|---|---|---|---|---|
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | Grade 3 | Grade 4 | |
| Gemcitabine/Vinorelbine | 4 | 0 | 18 | 5 | 6 | 2 |
| Vinorelbine | 1 | 0 | 14 | 3 | 4 | 1 |
| Cisplatin/Gemcitabine | 5 | 13.3 | 6.7 | 8.3 | 1.7 | |
| Cisplatin/Vinorelbine | 4.9 | 14.7 | 8.2 | |||
| Gemcitabine/Vinorelbine | 2 | 0 | 16 | 13 | 3 | <1 |
| Vinorelbine | 3 | <1 | 14 | 11 | <1 | |
| Gemcitabine | 2 | 7 | 1 | 2 | 1 | |
| Docetaxel | 2.3 | 1.1 | 26.1 | 56.8 | 0 | 0 |
| Vinorelbine | 8.8 | 1.1 | 30.8 | 38.5 | 0 | 0 |
Patient-Specific Factors and Management
It remains unclear why some older individuals are predisposed to myelotoxicity and others are not. Certainly, age-related changes occur in other organs and tissues other than bone marrow that may contribute to this predisposition, particularly with regard to alterations in clearance and pharmacodynamics of potentially myelotoxic chemotherapy drugs. Awareness of these changes and appropriate adjustments for individuals with a reduced capacity to metabolize or excrete an active drug can eliminate or reduce myelosuppressive toxicity.
Age-Related Physiological Changes
Changes in the Renal System
Age-associated changes in the kidneys including a decrease in glomerular filtration rate (GFR) and decreased concentrating ability predispose the elderly to a greater prevalence of chronic kidney disease, fluid and electrolyte imbalances, and impaired handling of drugs cleared by the kidneys with an increase in toxicity. It is estimated that GFR decreases at a rate of 1 mL/minute/year after the age of 40. Adjusting the dosage of drugs cleared by the kidneys may reduce the risk of toxicity. Assessing renal function using serum creatinine may be inaccurate as a result of decreased muscle mass in the elderly. An increased risk of hematologic toxicity was seen in older postmenopausal women with breast cancer and serum creatinine values of 1.5 mg/dL or less receiving adjuvant CMF compared to their younger counterparts. The creatinine clearance provides a more accurate estimate of renal function and can be used to predict toxicity. A retrospective study of 1,405 patients aged 65 years or older with breast cancer who were treated with CMF between 1998 and 2000 demonstrated increased hematological toxicity for those with a calculated creatinine clearance of less than 50 mL/min. Increased myelosuppression associated with renal insufficiency has been observed with melphalan, fludarabine, cisplatin, etoposide and topotecan in those older than 70. Dose modifications are recommended on the basis of creatinine clearance, particularly for elderly patients being treated with these drugs. Another prospective study in older breast cancer patients showed that hematological toxicity was substantially decreased by treating with modified dosing of cyclophosphamide and methotrexate on the basis of the estimated creatinine clearance. Many methods of calculating creatinine clearance are available, but the most commonly used is the Cockcroft-Gault formula, which calculates clearance on the basis of age and weight. However, this formula may also underestimate creatinine clearance in the elderly.
Changes in the Gastrointestinal System
Altered hepatic enzyme function leads to abnormalities in the metabolism of selected drugs. Decreased intracellular water, increased fat content, and low albumin in the elderly can significantly alter the volume and distribution of drugs. The pharmacokinetics and pharmacodynamics of the drugs may be influenced by their bound and unbound fractions. Both paclitaxel and docetaxel are extensively protein bound and are metabolized by the cytochrome P450 enzymes in the liver. No dose modification on the basis of age alone is recommended, but care should be exercised in elderly patients with indicators of poor nutritional status and who are on multiple drugs. Increased hematological toxicity due to altered gastrointestinal drug absorption secondary to age-associated decreased motility and decreased blood flow may be seen with oral cancer drug therapy.
Changes in the Renal System
Age-associated changes in the kidneys including a decrease in glomerular filtration rate (GFR) and decreased concentrating ability predispose the elderly to a greater prevalence of chronic kidney disease, fluid and electrolyte imbalances, and impaired handling of drugs cleared by the kidneys with an increase in toxicity. It is estimated that GFR decreases at a rate of 1 mL/minute/year after the age of 40. Adjusting the dosage of drugs cleared by the kidneys may reduce the risk of toxicity. Assessing renal function using serum creatinine may be inaccurate as a result of decreased muscle mass in the elderly. An increased risk of hematologic toxicity was seen in older postmenopausal women with breast cancer and serum creatinine values of 1.5 mg/dL or less receiving adjuvant CMF compared to their younger counterparts. The creatinine clearance provides a more accurate estimate of renal function and can be used to predict toxicity. A retrospective study of 1,405 patients aged 65 years or older with breast cancer who were treated with CMF between 1998 and 2000 demonstrated increased hematological toxicity for those with a calculated creatinine clearance of less than 50 mL/min. Increased myelosuppression associated with renal insufficiency has been observed with melphalan, fludarabine, cisplatin, etoposide and topotecan in those older than 70. Dose modifications are recommended on the basis of creatinine clearance, particularly for elderly patients being treated with these drugs. Another prospective study in older breast cancer patients showed that hematological toxicity was substantially decreased by treating with modified dosing of cyclophosphamide and methotrexate on the basis of the estimated creatinine clearance. Many methods of calculating creatinine clearance are available, but the most commonly used is the Cockcroft-Gault formula, which calculates clearance on the basis of age and weight. However, this formula may also underestimate creatinine clearance in the elderly.
Changes in the Gastrointestinal System
Altered hepatic enzyme function leads to abnormalities in the metabolism of selected drugs. Decreased intracellular water, increased fat content, and low albumin in the elderly can significantly alter the volume and distribution of drugs. The pharmacokinetics and pharmacodynamics of the drugs may be influenced by their bound and unbound fractions. Both paclitaxel and docetaxel are extensively protein bound and are metabolized by the cytochrome P450 enzymes in the liver. No dose modification on the basis of age alone is recommended, but care should be exercised in elderly patients with indicators of poor nutritional status and who are on multiple drugs. Increased hematological toxicity due to altered gastrointestinal drug absorption secondary to age-associated decreased motility and decreased blood flow may be seen with oral cancer drug therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



