Chemotherapy in the Treatment of Squamous Cell Carcinoma of the Head and Neck
Nabil F. Saba
Fadlo R. Khuri
INTRODUCTION
Cancers of the upper aerodigestive tract comprise a variety of malignancies with different sites of origin extending from the lips to the cervical esophagus, with squamous cell carcinoma (SCC) representing the most prevalent histology. Squamous cell carcinoma of the head and neck (SCCHN) is an aggressive malignancy affecting close to 52,000 patients yearly in the United States and leading to more than 300,000 yearly deaths worldwide.1 Tobacco smoking and alcohol use are known to be synergistic risk factors in the development of these cancers, which combined, accounted for approximately three-fourths of all SCCHN in the United States in the 1980s.2 The incidence and prevalence of human papillomavirus (HPV)-associated oropharyngeal cancers have increased in recent years.3,4,5,6 Multiple retrospective series have shown that patients with HPV(+) oropharyngeal squamous cell carcinoma (OPSCC) have a better prognosis than do patients with HPV(−) tumors.7,8 Recent data suggest that smoking has an adverse effect on prognosis for HPV-positive as well as HPV-negative disease, with the risk of death significantly increasing with each additional pack-year of tobacco smoking.9 HPV-positive disease also seems to have an unusual metastatic pattern.10
Although cancer of the head and neck represents only 5% of all newly diagnosed malignancies in the United States, it remains one of the most challenging to treat. Surgery and radiation therapy are two key components of the initial treatment of locally advanced SCCHN. Based on meta-analysis data, chemotherapy improves survival in patients treated for cure for nonmetastatic SCCHN with a higher benefit when used concomitantly along with radiotherapy.11 These trials included patients with cancer of the oral cavity, oropharynx, hypopharynx, and larynx. The role of induction chemotherapy prior to concurrent chemoradiotherapy remains controversial, even though the addition of a taxane to a platinum-based induction regimen has augmented the effectiveness of induction therapy.12,13 Although this finding has been confirmed by a recent meta-analysis,14 randomized trials have yet to prove the superiority of adding induction chemotherapy to the standard concurrent therapy approach.15,16
Cytotoxic chemotherapy was initially used primarily for palliation in the treatment of cancer of the head and neck. However, its use has gained increased acceptance as a primary and/or adjuvant modality over the past two decades and is now an integral part of the multidisciplinary management of SCCHN. As the objectives of chemotherapy in this field have changed dramatically, systemic therapy has such wide-ranging applications as radiation sensitization and chemoprevention. This chapter reviews the historical usage and current role for chemotherapy in the management of SCCHN.
TREATMENT OF NEWLY DIAGNOSED CANCER—GENERAL APPROACHES
Treatment of SCCHN is complicated by the diversity of cancer sites and origins, the vital anatomic structures within the treated area, and the need for preservation of organ function. The ideal approach to therapy involves a multidisciplinary team of surgeons, radiation and medical oncologists, dieticians, speech pathologists, and dentists. Approximately 30% to 40% of patients with SCCHN present with early-stage cancer manageable with either primary surgery or definitive radiation therapy. In these patients, despite the curative nature of these treatment modalities, the risk of a second primary malignancy is significantly increased compared with the age-matched general population.17,18 Smoking cessation is of primary importance in reducing the incidence of second primary cancers, and chemoprevention to reduce recurrence rates of index cancers and to prevent the occurrence of second primary cancers in the aerodigestive tract has been a topic of interest and substantial research effort.19 Unfortunately, none of the chemopreventive agents studied to date in patients who completed definitive therapy for early-stage disease has shown a clear benefit in prospective randomized clinical trials (please refer to the section of Chemoprevention in this chapter for a more detailed account of this topic).
Even though local therapeutic interventions remain a key component for the treatment of locally advanced SCCHN, chemotherapy has earned a significant role and is now an essential component of managing locally advanced cancer.20 Treatment planning, including decisions about the use of chemotherapy, should be made within the setting of a multidisciplinary approach. Although functional organ preservation for patients with cancers of the oropharynx, larynx, and hypopharynx has been widely adopted, using nonsurgical approaches in many instances, this still does not apply to oral cavity malignancies where management still relies heavily on surgical resection. Some studies have suggested an added benefit for less extensive surgery when chemotherapy is used for oral cavity cancers, yet this has not been proven in large randomized clinical trials.21,22
CONCOMITANT CHEMORADIOTHERAPY
Concurrent chemoradiotherapy significantly decreases the risk of death compared with definitive radiotherapy alone based on the meta-analysis of chemotherapy in cancer of the head
and neck (MACH-NC) study, which revealed a 6.5% total decrease in 5-year mortality with the addition of chemotherapy.11 Although the meta-analysis confirmed a greater benefit with platinum-based chemotherapy, the optimal regimen in the concurrent setting is still a point of debate. High-dose, single-agent cisplatin administered at 100 mg/m2 on days 1, 22, and 43 in concurrence with conventional single daily fractionated radiation therapy has been considered the standard systemic regimen.23 However, the improved survival noted with this regimen was also associated with increased toxicities, both acute and chronic.23,24 It is unclear whether a third cycle of cisplatin would have made a significant difference in outcome. The Radiation Therapy Oncology Group (RTOG) 0129 study randomized patients to once-daily radiation fractionation with three cycles of cisplatin in comparison to accelerated boost administered over a total of 6 weeks with two cycles only. No significant difference in survival was observed between the two arms. More recent cooperative group trials have restricted cisplatin to two cycles if administered with a concomitant boost approach.
and neck (MACH-NC) study, which revealed a 6.5% total decrease in 5-year mortality with the addition of chemotherapy.11 Although the meta-analysis confirmed a greater benefit with platinum-based chemotherapy, the optimal regimen in the concurrent setting is still a point of debate. High-dose, single-agent cisplatin administered at 100 mg/m2 on days 1, 22, and 43 in concurrence with conventional single daily fractionated radiation therapy has been considered the standard systemic regimen.23 However, the improved survival noted with this regimen was also associated with increased toxicities, both acute and chronic.23,24 It is unclear whether a third cycle of cisplatin would have made a significant difference in outcome. The Radiation Therapy Oncology Group (RTOG) 0129 study randomized patients to once-daily radiation fractionation with three cycles of cisplatin in comparison to accelerated boost administered over a total of 6 weeks with two cycles only. No significant difference in survival was observed between the two arms. More recent cooperative group trials have restricted cisplatin to two cycles if administered with a concomitant boost approach.
For patients who are not candidates for high-dose cisplatin, a weekly cisplatin regimen has been used with cisplatin doses ranging from 30 to 50 mg/m2. Although considered to be reasonable options by many, these approaches have not been tested in a randomized prospective fashion in comparison with the every 3 weeks platinum regimen.
Alternative regimens in the case of contraindications to cisplatin include combinations of carboplatin and 5-fluorouracil (5-FU).25 Taxane-based regimens with the most commonly used weekly paclitaxel and carboplatin have also been considered as acceptable alternatives.26 Limitations of these approaches have been the lack of agreement on the best taxane schedule and dose and the lack of randomized clinical trials comparing these regimens to cisplatin. A recent meta-analysis comparing the two approaches revealed no clear advantage of cisplatin over the taxane-based regimens that include carboplatin.27
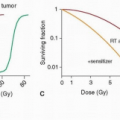
Concomitant chemoradiotherapy has been investigated over the past four decades as a primary treatment approach in locally advanced cancer of the head and neck. It is administered with the intent of curing locoregional cancer and controlling the occurrence of distant metastases. Theoretically, systemic control may be feasible if the dose of chemotherapy administered is equivalent to standard systemic doses when given in combination with radiotherapy. Chemotherapy should also act as a radiation sensitizer (by improving the tumoricidal activity of radiation) or as an enhancer (with direct cytotoxic properties against the primary tumor). Therefore, combined chemoradiotherapy provides potentially increased antitumor activity, although often at the risk of substantial local toxicity.
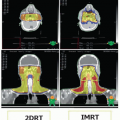
Generally, radiation therapy is administered in two basic schedules: concomitant (simultaneous) or in an interrupted fashion (alternating or split-course schedule). The schedule of radiation delivery may have an impact on both treatment outcome and the incidence of acute and chronic toxicities. A phase III trial (RTOG 9003) enrolled more than 1,000 patients with locally advanced SCCHN28 to four arms, including1 standard radiotherapy,2 hyperfractionated twice-daily radiotherapy,3 accelerated fractionated twice-daily therapy, and4 accelerated fractionated therapy with concomitant boost. After a median follow-up of 23 months, it was determined that hyperfractionated or accelerated radiation therapy with boost provided increased locoregional control and a trend toward improved disease-free survival (DFS) in comparison with conventional radiation therapy. However, no improvement was noted in overall survival (OS). Patients given accelerated split-course fractionation had outcomes similar to those who had received conventional radiotherapy. Clinical investigators continue to incorporate accelerated, fractionated, hyperfractionated, and intensity-modulated radiotherapy (IMRT) approaches in an attempt to maximize tumoricidal activity while minimizing associated toxicities.
Earlier studies examined single-agent chemotherapy with concomitant daily radiotherapy. Cytotoxic agents used in the palliative setting for recurrent or advanced disease have demonstrated single-agent activity when combined with radiation therapy. Frequently administered single agents included cisplatin, methotrexate, 5-FU, bleomycin, ifosfamide, and the taxanes. Several randomized studies have been completed, demonstrating the benefits of combined therapy in comparison with radiation therapy alone. An early randomized trial of radiotherapy alone versus radiotherapy with bolus 5-FU29 clearly demonstrated superior local control and survival with the addition of 5-FU to radiotherapy in patients with cancer of the oral cavity and oropharynx. Other studies demonstrated the enhanced radiosensitization properties of 5-FU when administered continuously for at least 48 hours following radiation therapy.30 The continued exposure to 5-FU (via continuous infusion rather than bolus) following radiation therapy seemed to provide superior radiosensitization.30 On the basis of this principle, a phase I/II pilot study was conducted to study the effects of dose-escalating continuous infusion 5-FU (20 to 30 mg/kg in 5-mg/kg increments) over a 5-day period with four sequential daily fractions of radiotherapy (2.5 Gy) on days 1 to 4, repeated every 14 days.31 A complete response (CR) rate of 75% was attained in stage IV patients.
In addition, based on preclinical animal models and promising results from earlier studies, hydroxyurea was approved by the FDA for use in patients with SCCHN when administered concomitantly with radiotherapy.32 Hydroxyurea has not gained widespread use in concurrence with radiation therapy despite its common use in a few centers such as the University of Chicago. Earlier studies of bleomycin examined its potential synergistic activity in combination with radiotherapy. Two previous randomized studies involving more than 200 patients suggested improved locoregional control in the combined chemoradiation arm.33 However, the European Organisation for Research and Treatment of Cancer (EORTC) could not confirm benefit of response after completing a randomized study of conventional radiotherapy with or without singleagent bleomycin.34 Only 64% of patients in the chemoradiation arm received the recommended dose, which may have contributed to the suboptimal response rate and survival time in the combined chemoradiation arm.
The platinum analogues, carboplatin and cisplatin, have a well-defined role in combination with radiation therapy. A large phase III European study evaluated the benefits of concomitant cisplatin (100 mg/m2, days 1, 22, and 43) with radiation therapy.35 The study randomly assigned 334 patients to daily radiation therapy or chemoradiotherapy after surgical resection. After a median follow-up of 60 months, the rate of progression-free survival (PFS) was significantly higher in the combined therapy group than in the group given radiotherapy alone (p = 0.04). The OS rate was also significantly higher in the combined therapy group than in the radiotherapy
group (p = 0.02), confirming that postoperative concurrent administration of high-dose cisplatin with radiotherapy is more efficacious than is radiotherapy alone in patients with locally advanced cancer of the head and neck and does not cause an undue number of late complications.
group (p = 0.02), confirming that postoperative concurrent administration of high-dose cisplatin with radiotherapy is more efficacious than is radiotherapy alone in patients with locally advanced cancer of the head and neck and does not cause an undue number of late complications.
Several similar studies have confirmed the advantage of platinum-based concurrent therapy over radiation therapy alone as a definitive therapy for locally advanced SCCHN.11,36,37,38 The platinum agent of choice remains cisplatin despite the equivalent results observed in small international trials. Other potential chemosensitizing agents that remain largely investigational include carboplatin and docetaxel.39,40 The toxicity profile of other agents such as gemcitabine has precluded development in this setting.41
Several studies have explored the use of multiagent chemotherapy in concurrence with radiation. Early studies investigated the platinum 5-FU regimen and revealed encouraging results.38,42,43,44,45 In all, more than 70 randomized trials have compared radiation alone with chemoradiotherapy. Several of these studies involved small cohorts of patients.11 An example of a study using concurrent cisplatin and 5-FU is the Cleveland Clinic study that enrolled 222 patients with locally advanced SCCHN and administered 96-hour continuous infusion of cisplatin (20 mg/m2/day) and fluorouracil (1,000 mg/m2/day) with standard or fractionated radiation.38 With a median follow-up of 73 months, a 5-year OS rate of 65.7% and a local control rate of 86.7% were observed. The toxicity profile was similar to that of bolus-dose cisplatin with radiation therapy: 78.4% of patients required nutritional support via PEG tube, and one died from a pulmonary embolism.
The MACH-NC meta-analysis, which evaluated a total of 87 trials and 16,485 patients, showed an absolute benefit for chemotherapy of 4.5% at 5 years and a significant interaction (p < 0.0001) between chemotherapy timing (adjuvant, induction, or concomitant) and treatment. Both direct (six trials) and indirect comparisons showed a more pronounced benefit of the concomitant chemotherapy as compared to induction chemotherapy.11
More recently, investigators and cooperative groups have placed increasing focus on the exploration of less intensive regimens with lower end-organ toxicities. This has been accelerated by the finding that HPV status is a strong and independent prognostic factor for survival among patients with cancer of the oropharynx9 and by the increased incidence of HPV-related oropharyngeal SCC predominantly in Caucasian males.3,46
The use of noncisplatin taxane-based regimens in the concurrent setting remains an attractive secondary option for patients who are not candidates for cisplatin therapy, despite the lack of randomized clinical trials in this area. A regimen of weekly carboplatin (100 mg/m2) and paclitaxel (45 mg/m2) and concurrent radiotherapy of 70.2 Gy resulted in a CR rate of 75% with a median OS of 33 months that was higher in complete responders with OS rates of 79% and 61% at 2 and 3 years follow-up, respectively.47 Even though significant acute toxicities were reported, with 70% of patients experiencing grade 3 mucositis, 30% leukopenia, and 25% skin desquamation. However, the ototoxicity and nephrotoxicity typically seen with cisplatin use were not observed. In a second phase II trial, weekly carboplatin with an area under the curve (AUC) of 1 with paclitaxel at 45 mg/m2 was given with 69.6 Gy of daily radiotherapy to the primary cancer. The primary toxicities included grade 3 or 4 stomatitis, dysphagia, and mucositis in 55% of patients; however, the regimen was effective with an overall response rate (ORR) of 84%, CR rate of 67%, and an OS rate of 60% at a median follow-up of 36 months.48 Taxane-based regimens remain a very rational approach for patients with contraindications to cisplatin-based therapy. However, no clear randomized trials have established a therapeutically equivalent regimen to the standard cisplatin-based therapy. A recent meta-analysis comparing the two approaches was recently reported27 and revealed at least equivalent results with the use of taxane-based regimens. The optimal dose and schedule of paclitaxel and carboplatin remain, however, poorly defined.
SEQUENTIAL THERAPY
Induction chemotherapy is administered in a sequential fashion before the provision of definitive surgery and/or radiation therapy. The goal of induction therapy is to assist in both local and distant cancer control. Theoretically, this is done by reducing overall tumor burden before the definitive therapy, which ultimately allows organ preservation and function and possibly improved quality of life. Distantly, the systemic effects of induction chemotherapy may prevent the dissemination of microscopic disease eventually promoting OS. In addition, an improvement in the chance of organ preservation such as preservation of the larynx and hypopharynx is possible, which would have a profound positive impact on quality of life. With the increasing toxicity noted with concurrent therapy schedules, and with the improvement in locoregional control observed with the use of concurrent therapy, interest in curtailing distant metastases as a significant cause of mortality has gained increased importance;49 hence the concept of sequential therapy, which combines both induction and concurrent therapy approaches. In the MACH meta-analysis, induction chemotherapy offered a meager advantage of 5% improvement in survival over radiation alone; however, this was observed with platinum-based regimens that did not include taxanes.50,51 Since then, randomized clinical trials comparing induction chemotherapy with cisplatin and 5-FU and a taxane (docetaxel or paclitaxel) showed a clear advantage to adding a taxane in the induction setting and led to the approval of Docetaxel, Cisplatin, 5FU (TPF) as the induction regimen of choice for locally advanced SCCHN12,13 (Table 31.1).
In achieving the desired goals of induction chemotherapy, combination treatment with platinum-based 5-FU has been the traditional approach based on its earlier success in recurrent and advanced cancer. Review of the literature suggests that all studies incorporating induction chemotherapy have failed to provide improved locoregional control or a benefit in OS.
Three landmark phase III trials have established the benefits of induction chemotherapy in laryngeal preservation. The Veterans Affairs Laryngeal Study randomly assigned 332 patients with stage III or IV laryngeal carcinoma to receive three cycles of cisplatin/5-FU (PF) followed by conventional radiotherapy or laryngectomy followed by conventional radiotherapy.52 Response was assessed after the completion of two cycles of chemotherapy. Patients with a partial response (PR) received a third cycle of chemotherapy followed by radiotherapy. In contrast, nonresponders immediately underwent a laryngectomy followed by radiation therapy. An integral component of this study was salvage surgery, which was offered to
all patients with residual disease at the completion of radiotherapy. After two cycles of induction, the ORR was 85% (31% CR, 54% PR). Histologic specimens were obtained in 103 patients at the completion of chemotherapy, validating a complete response in 88% of patients with a clinical CR; 45% of those presumed to have a clinical PR were confirmed histologically. Overall, 64% of patients had a histologically confirmed CR. After a median follow-up of 33 months, the estimated 2-year survival was 68% in both treatment groups, and there was no difference in OS (p = 0.9846). However, preservation of the larynx was maintained in 64% of patients. Patterns of recurrence differed between the two groups, with increased locoregional disease failure (p = 0.0005) but decreased metastases (p = 0.016) in the induction chemotherapy group. Although there was no significant difference in OS, this study demonstrates that induction chemotherapy is feasible in the setting of cancer of the larynx, allowing organ preservation without compromising OS. It should be noted that of the 166 patients on the chemotherapy arm, 120 patients (72%) had N0 or N1 disease.
all patients with residual disease at the completion of radiotherapy. After two cycles of induction, the ORR was 85% (31% CR, 54% PR). Histologic specimens were obtained in 103 patients at the completion of chemotherapy, validating a complete response in 88% of patients with a clinical CR; 45% of those presumed to have a clinical PR were confirmed histologically. Overall, 64% of patients had a histologically confirmed CR. After a median follow-up of 33 months, the estimated 2-year survival was 68% in both treatment groups, and there was no difference in OS (p = 0.9846). However, preservation of the larynx was maintained in 64% of patients. Patterns of recurrence differed between the two groups, with increased locoregional disease failure (p = 0.0005) but decreased metastases (p = 0.016) in the induction chemotherapy group. Although there was no significant difference in OS, this study demonstrates that induction chemotherapy is feasible in the setting of cancer of the larynx, allowing organ preservation without compromising OS. It should be noted that of the 166 patients on the chemotherapy arm, 120 patients (72%) had N0 or N1 disease.
Table 31.1 Major Randomized Phase III Trials of Induction Chemotherapy in Treating SCCHN | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
In the RTOG 9111 study, a total of 547 patients with stage III/IV laryngeal carcinoma53 were randomized to three cycles of induction chemotherapy of cisplatin/5-FU followed by daily radiation therapy, concurrent cisplatin (100 mg/m2, days 1, 22, and 43) and radiation therapy, or daily radiation therapy only (70 Gy). The primary endpoint was laryngeal preservation rather than OS. A preliminary analysis of laryngectomy-free survival at 2 years showed improvement in both the chemotherapy-containing arms, but notably so in the concurrent chemotherapy arm (58% vs. 66%). Although DFS appeared to be favorable in both chemotherapy arms in comparison with radiation therapy only, no clear difference in OS was noted among the three arms of the study. Time to laryngectomy was superior in the concurrent chemotherapy arm in comparison with the induction arm (p = 0.0094). These recently updated 10-year results continue to show that induction PF followed by RT and concomitant cisplatin/RT shows similar efficacy for the composite endpoint of laryngectomy-free survival. Locoregional control and larynx preservation were significantly improved with concomitant cisplatin/RT compared with the induction arm or RT alone.53 Hence, it can be concluded that despite variation in methods of treatment, neither has provided an advantage in OS despite the trends favoring induction chemotherapy. Nonetheless, the results from this trial suggest a prolonged DFS and laryngectomy-free interval with the addition of chemotherapy to radiation therapy.
The EORTC verified the benefits of organ preservation in a randomized phase III clinical trial in patients with stage III/IV cancer of the pyriform sinus or aryepiglottic folds.54 One hundred ninety-four eligible patients were randomly assigned to immediate surgery followed by radiotherapy (94 patients) or induction chemotherapy (100 patients) with cisplatin (100 mg/m2, day 1) and continuous-infusion 5-FU (1,000 mg/m2/day, days 1 to 5). An endoscopic examination was completed after each cycle. Patients with a CR or a PR following cycle 2 were offered a third cycle of chemotherapy. Unlike the Veterans Affairs Laryngeal Cancer Study, patients were required to achieve a CR before undergoing radiation therapy; patients with stable disease were offered salvage surgery followed by radiotherapy. Induction chemotherapy resulted in a CR in 54% of patients at the primary site, and 51% of patients achieved a locoregional CR. Overall, induction chemotherapy provided fewer distant metastases (p = 0.041) and an improved median survival (44 months) versus the surgical arm (25 months). Unfortunately, neither arm demonstrated superiority in the prevention of locoregional recurrence. Treatment with induction chemotherapy managed to preserve the larynx in 42% and 35% of patients evaluated for 3- and 5-year estimates of survival. This European study suggests that induction chemotherapy is a feasible alternative if organ preservation is desired without compromising OS. However, it should be noted that only 31% of patients had N2/N3 disease, of which only 6% were N3. Furthermore, patients with N3 disease were eventually excluded from this trial because the first six patients failed to achieve a CR following induction.54 The updated 10-year results of the EORTC trial 24891 continue to show that more than half of the survivors retain their larynx without compromising survival.55
Despite the reduction in distant metastases observed in a number of trials using the induction or sequential approach, the superiority of these approaches over the standard concurrent regimen has yet to be proved in a randomized phase III trial.
Sequential and concurrent therapy were compared head to head in a phase II randomized clinical trial in 101 patients with stage III to IV SCCHN. Patients randomized to concurrent therapy received two cycles of cisplatin plus 5-FU in combination with radiation therapy. Patients assigned to the sequential therapy arm first received three cycles of docetaxel, cisplatin, and 5-FU, followed by the same chemoradiation therapy. The complete radiologic response rate was clearly superior in the sequential arm with an accompanying increase in OS and PFS, providing substantial support to the proponents of induction therapy.56
Unfortunately, these results could not be reproduced in two US-based randomized phase III studies comparing the
sequential to the concurrent approach (Table 31.1). In the PARADIGM trial, 145 patients were randomized to sequential versus concurrent therapy. The schema of the sequential arm was, however, complicated and was dependent on the response to induction TPF. Docetaxel was chosen as the concurrent agent of choice with a concomitant boost radiotherapy given to patients with less than good response to induction therapy, whereas standard radiation with concurrent carboplatin was used for the good responders. The trial fell short of completing accrual and accrued <50% of its intended goal. No sign of any benefit from sequential therapy on overall outcome was observed.15
sequential to the concurrent approach (Table 31.1). In the PARADIGM trial, 145 patients were randomized to sequential versus concurrent therapy. The schema of the sequential arm was, however, complicated and was dependent on the response to induction TPF. Docetaxel was chosen as the concurrent agent of choice with a concomitant boost radiotherapy given to patients with less than good response to induction therapy, whereas standard radiation with concurrent carboplatin was used for the good responders. The trial fell short of completing accrual and accrued <50% of its intended goal. No sign of any benefit from sequential therapy on overall outcome was observed.15
The DECIDE trial randomized patients to induction with two cycles of docetaxel, cisplatin, and 5-FU followed by chemoradiotherapy with docetaxel, 5-FU, and hydroxyurea in concurrence with radiation therapy. There was no noted difference in PFS or OS.16 Febrile neutropenia was also more frequent on the induction arm. Of note is that both of these phase III studies suffered from lack of reaching their accrual targets and an overly pessimistic prediction of outcome for the control arm in the era of HPV-positive OPSCC.
CANCER OF THE NASOPHARYNX
Nasopharyngeal cancer (NPC) is staged according to the International Union Against Cancer (UICC) and the American Joint Committee on Cancer (AJCC) criteria57,58 and classified into three subtypes based on the World Health Organization (WHO) criteria: type I or keratinizing, type II or differentiated nonkeratinizing, and type III or undifferentiated, which is usually associated with the EBV virus.59,60
It is unclear if systemic therapy in the adjuvant setting or primary therapeutic setting has any value in patients with early-stage NPC given the paucity of clinical trials examining this question. For stage II or intermediate-stage cancer, concurrent therapy is considered a valid approach given the results of a randomized trial favoring the concurrent approach to single-modality radiation therapy. A total of 1,992 patients with stage II NPC were randomized to weekly cisplatin and radiotherapy versus radiation alone. Better OS and PFS were observed on the concurrent therapy arm. In addition, the rate of metastases was more favorable in the concurrent arm.61
Concurrent chemoradiotherapy remains the mainstay for the treatment of locally advanced NPC. An early RTOG study opened the door for exploring concurrent modality therapy as the treatment of choice for locally advanced NPC. In a phase II study comparing concurrent radiotherapy plus cisplatin (100 mg/m2, days 1, 22, and 42) to standard radiotherapy alone in 124 patients with locally advanced SCCHN,62 patients with NPC receiving concurrent treatment had an impressive CR of 89% in a subset analysis. Comparison with historically matched controls revealed that DFS and OS were greater in the concomitant arm, thereby revolutionizing the standard treatment of NPC. Subsequently, the large phase III intergroup study 00 to 99 of daily radiotherapy and cisplatin (100 mg/m2, days 1, 22, and 43) followed by three cycles of adjuvant cisplatin (80 mg/m2, day 1) and continuous-infusion 5-FU (1,000 mg/m2, days 1 to 4) every 28 days revealed a clear advantage of the concomitant arm with a 3-year DFS of 69% versus 24% (p < 0.001) and a 3-year OS of 78% versus 47% (p = 0.005).63
Even though the results of the phase III intergroup 00 to 99 study were confirmed in subsequent trials,64 the acute and late toxicities associated with this regimen have led to the exploration of other effective and less toxic chemotherapeutic combinations. Carboplatin, which is deemed to be less toxic, was compared head to head with cisplatin in this setting with similar efficacy and reportedly lower toxicity. Still, more studies are needed to confirm these preliminary findings.65
Given the difficulty in completing the therapeutic regimen on the intergroup 00 to 99 study, an increased interest has developed in sequential therapy for NPC. Patients with stages III and IV disease were randomized to sequential versus concurrent therapy. Although the results were encouraging, the sequential group had a different fractionation schedule for radiation and a higher incidence of toxicities,66 begging for more studies to investigate this question.
A meta-analysis of six randomized trials evaluating chemoradiation therapy (induction, concomitant, and adjuvant) versus radiation therapy alone verified that the addition of chemotherapy to radiation therapy increased DFS by 37% at 2 years, 40% at 3 years, and 34% at 5 years. The addition of chemotherapy to standard radical radiation therapy for locoregionally advanced cancer of the nasopharynx increases both disease-free/progression-free and OS by 19% to 40% at 2 to 4 years after treatment.67 In contrast, in a large single-institution study in Hong Kong, 240 patients were randomly assigned to radiation therapy or to two or three cycles of induction chemotherapy (cisplatin 60 mg/m2 and epirubicin 110 mg/m2) followed by radiation therapy.68 After a median follow-up of 71 months, the investigators determined no statistical benefit with induction chemotherapy in nodal relapse-free survival (p = 0.13), prevention of distant metastases (p = 0.56), or survival (p = 0.55). Despite these results, the addition of chemotherapy is the overwhelmingly accepted standard of care for treating locoregionally advanced NPC.
Systemic therapy is considered the mainstay of care for patients with advanced or recurrent NPC who are not candidates for local therapeutic interventions. In this setting, combination therapies seem to produce superior results when compared to single-agent therapies.69 Several regimens appear to have equivalent results; however, there is a clear paucity of randomized clinical trials comparing different agents in this setting.70 EBV DNA levels measured in plasma are reported to predict overall outcome of patients with recurrent metastatic NPC and may be used as a method to assess response and predict outcome.71 Recently, markers of cisplatin sensitivity such as polymorphism of ERCC1 C8092A were found to be predictors of outcome in cisplatin-treated patients.72
POSTOPERATIVE THERAPY
In addition to concurrent or sequential chemoradiotherapy, surgical resection followed by postoperative therapy guided by pathologic findings is considered a standard definitive therapeutic approach in SCCHN. Local and distant recurrences remain frequent after surgery for locally advanced cancer, which presses the need for improved postsurgical adjuvant approaches.77,78 Radiotherapy in the postoperative setting has been delivered to patients with resected stages III, IVa, and IVb disease with specific indications including surgical margin
involvement, perineural and lymphovascular involvement, bone or cartilage invasion, extracapsular lymph node extension, advanced T3 or T4 primary tumors, and N2 or N3 lymph node metastases. This approach has resulted in a 5-year survival rate of close to 30% to 40%.35,79,80 The addition of systemic chemotherapy has been shown to improve locoregional as well as overall and disease-free survival in patients with high-risk features.79,81
involvement, perineural and lymphovascular involvement, bone or cartilage invasion, extracapsular lymph node extension, advanced T3 or T4 primary tumors, and N2 or N3 lymph node metastases. This approach has resulted in a 5-year survival rate of close to 30% to 40%.35,79,80 The addition of systemic chemotherapy has been shown to improve locoregional as well as overall and disease-free survival in patients with high-risk features.79,81
Cisplatin at 100 mg/m2 added to 60 to 66 Gy of postoperative radiotherapy was tested in trials by both EORTC and RTOG.35,79 The studies had notable differences in their inclusion criteria as well as their primary objectives, which were locoregional control in the RTOG study and PFS in the EORTC study. Adjuvant concurrent therapy was associated with an improved 5-year PFS (47% vs. 36%, p = 0.04) and OS (53% vs. 40%, p = 0.02) as well as a lower rate of locoregional recurrence (18% vs. 31%, p = 0.007) and a longer time to progression (55 vs. 23 months) on the EORTC trial. This came at a higher toxicity price on the concurrent arm with a significant difference in acute mucositis (41% vs. 21%). On the RTOG study, despite the improved locoregional control and DFS observed in the initial 3-year analysis, the updated 5-year results revealed a loss of significant difference in DFS (37.4% vs. 29.1%, p = 0.098) and locoregional control (79.5% vs. 71.3%, p = 0.086). It is of note that in both studies, cisplatin did not influence the rate of distant metastases, which were 21% versus 25% in the EORTC study (p = 0.61) and 23% versus 21% in the RTOG trial (p = 0.46), for the radiation alone arm and concurrent arm, respectively.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree