Fig. 10.1
Severity index for categorizing medication errors from NCC MERP (Used with permission. © 2001 National Coordinating Council for Medication Error Reporting and Prevention)
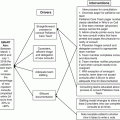
Classification of Processes Involved in Medication Errors
The processes that lead to medication errors related to chemotherapy mimic those involved in general medication errors. With the unique complexity and toxicity of these medications, however, the risk to the patient can increase greatly when an error does occur [7]. The areas in which chemotherapy medication errors can occur include the time of prescribing, preparing, dispensing, administering, and monitoring a medication (e.g., see Table 10.1). In many chemotherapy errors, there are numerous processes at play that contribute to the ultimate error taking place. Even within one location, there may be many issues that contribute to an error. With respect to prescribing errors, many medical facilities continue the practice of ordering chemotherapy via paper utilizing handwritten orders as opposed to computerized physician order entry (CPOE). This process can lead to numerous potential errors that may have been caught and otherwise eliminated by utilizing the process checks built into CPOE such as elimination of hand-writing interpretation errors and utilities such as dose calculators [8]. Coupled with severity classification, identifying and appropriately labeling the various processes involved in each error can help ensure accurate measurement and subsequent interpretation of the events that lead to a chemotherapy error.
Table 10.1
The processes where errors can occur while a patient receives chemotherapy and common errors associated with each procedure
Error location | Examples of errors |
|---|---|
Prescribing | Wrong dosing weight and height are used while calculating chemotherapy dose (mg/m2 vs. mg/kg) Wrong unit of measure utilized (milligram vs. microgram) Incorrect or absent dose adjustment based on prior toxicity |
Preparing | Incorrect diluent selected Prepared with wrong volume |
Dispensing | Product labeled incorrectly (wrong patient, wrong drug) Lack of verification that correct drug, diluent, and dose have been prepared by chemotherapy pharmacist |
Administration | Administered to wrong patient Infused over incorrect time (IV push vs. 3-h infusion) Administered via inappropriate route (IV vs. IT) Infusion pump is programmed incorrectly |
Monitoring | Failure to identify toxic levels of a drug Inappropriate monitoring for acute toxicities after administration of a drug (e.g., anaphylaxis) |
Medication Error Measuring Systems
In 1999, the Institute of Medicine published a report that outlined significant patient safety concerns through the medical system in the United States and outlined recommendations on how to measure and address these concerns [9]. The report elucidated that voluntary reporting systems have the best means to focus on patient safety improvement in the field of medication safety. These types of systems usually evaluate errors that resulted in minimal or no harm to patients, and analyzing these errors can lead institutions to identify and address vulnerabilities in their systems before harm occurs [9, 10]. Following the release of this report, there was a large growth and advancement in medication error reporting systems. One recent review cited over 12 various types of medication error reporting systems available [11]. Since that time with the help of technological advances within electronic medical record (EMR) information systems, automated medication event reporting systems have been implemented as well. With the many variations on measuring and reporting systems, medical institutions are now burdened with identifying which systems can help provide a balance between resources available and identifying patient safety concerns. Outlined in the following sections are the most commonly used reporting systems and analysis of the benefits and potential concerns of each.
Incident Reporting Systems
Incident reporting systems utilize structured data collection to input medication error information. These systems are all confidential and can vary from anonymous to non-anonymous reporting strategies. [12] Incident reporting systems are the most commonly utilized measurement system, and numerous reporting systems exist in healthcare in both local hospital systems and nationally [13]. Several countries have developed national adverse medication event systems, such as the UK’s National Reporting and Learning System (NRLS), which have allowed findings to be applied to a wider system and have a national affect [14]. On average over 1.5 million incident reports are submitted each year to the NRLS. In the United States, the Patient Safety and Quality Improvement Act of 2005 established a voluntary reporting system that utilizes patient safety organizations through the Agency for Healthcare Research and Quality in an attempt to standardize and nationalize incident reporting [15]. The benefits and concerns of these varied reporting systems can be applied fairly universally. These systems have been identified as being relatively easy to implement and are generally of low cost to a health system [11]. This system allows frontline staff who were directly involved in the incident to input data in a structured format that is submitted to be reviewed via the hospital systems’ designated review structure. Examples of standardized systems include MEDMARX and the MedWatch program from the US Food and Drug Administration (FDA) [16, 17]. Table 10.2 outlines the components required for documentation of an incident report using the MedWatch system from the FDA.
Table 10.2
Components required in documentation of incident report as outlined from MedWatch from the US FDA
Category | Details |
|---|---|
Patient information | Patient identifier (MRN/FIN), age, sex, height, weight, ethnicity/race |
Type of event | Adverse event, product defect |
Outcome | Death, life-threatening, hospitalization—initial or prolonged, other serious, required intervention to prevent permanent impairment/damage, disability or permanent damage, congenital anomaly/birth defects |
Chronology/location | Date, time, location/hospital unit |
Event description | Free text area for thorough event description |
Relevant tests/laboratory data | Any additional testing necessary secondary to the event that would not have been obtained otherwise |
Suspected medication | Name, dose, route, frequency, length of infusion time |
Indication for use | Diagnosis or problem indicated for use of medication |
While this type of measurement system may be easily implemented at relatively low cost, there exist several concerns regarding its stand-alone efficacy. Volunteer incident reporting systems can be impacted significantly by reporter bias [12, 18, 19]. They have been shown to identify only a small percentage of target problems and are dependent upon the involved parties accurately filling out details that are critical for analysis of the event [19]. An additional concern is that physicians have relatively low error reporting rates. One study which surveyed over 1600 hospitals reported that at 86% of hospitals, physicians submitted few or no incident reports [18]. With the complexities of chemotherapy and biotherapies in the practice of pediatric hematology/oncology and bone marrow transplant, physician contribution to error reporting is critical. While these concerns can overlap with many of the other reporting systems, a more specific concern is that events are reported without clearly identifying the total numbers of patients at risk for such an event [19]. This may allow for analysis of trends of error types over time, but does not allow for assessment of which populations are at most risk and who would benefit the most from intervention based on incident report review. Medication error measurement via incident report submission and review involves having a structured review process. Often multidisciplinary teams are involved in the review process, identification of potential preventable errors, and creation of subsequent system changes. This system of a review and subsequent feedback loop to correct and prevent errors is a critical element of all measurement systems.
Chart Review
Utilizing chart review as a form of medication error measurement builds upon many of the processes involved with incident report creation. Chart review does this in a retrospective manner in surveying patient’s medical records prior to an error being reported and attempting to identify issues in a timely manner [11, 20]. The process of chart review often involves evaluating many components of the medical record including medication administration record (MAR) review and identifying any specific signals or triggers that might be concerning for an error. While reviewing the patient’s MAR, the orders are screened for the appropriate inclusion of important details such as legibility, medication name, dosage form, route of administration, dose, dosage unit, frequency of administration, duration of therapy, number of doses to be dispensed, and directions or warnings for use [21, 22]. Data reviewers are vigilant for certain changes in patient status (e.g., transfer to the intensive care unit), and new diagnostic or laboratory tests that can indicate where errors may have occurred (e.g., abnormal echocardiogram, elevated liver enzymes). The data is collected on forms, and when errors are identified, further drill down occurs. This is a labor- and resource-intensive process that requires dedicated teams for review and usually necessitates daily review of charts to have effective real-time identification of errors and interventions.
In comparison to incident reports alone, more detailed data is generally gathered from prospective chart review [11, 23]. This system can be an effective tool at identifying errors during the prescribing, administration, and monitoring of medications [11, 22, 23]. As chart review identifies errors that have not yet been voluntarily reported, there have been concerns that the seriousness of problems detected via this method is often associated with lower clinical significance [23]. While a higher number of errors may be identified, fewer error reviews that would lead to system changes have been observed in some studies [22, 23]. The significant time and resources needed to have a large-scale prospective chart review for medication error must also be considered when evaluating this as a medication error measurement tool and often preclude this from being a viable solution for most institutions.
Trigger Tools
In 1999, the Institute for Healthcare Improvement’s expert panel on patient safety devised a trigger tool methodology that utilizes identifying key triggers in patient records during review that prompt medication error detection [24].The IHI Global Trigger Tool is incorporated by utilizing a team of three or more reviewers evaluating randomly selected charts and searching for triggers to adverse events in six modules: cares, medication, surgical, intensive care, perinatal, and emergency department [25].Within the medication module, triggers have been identified that are often included when a medication error occurs such as diphenhydramine administration, partial thromboplastin time (PTT) greater than 100 s, and vitamin K administration. The use of this trigger-focused review expands on the concept of chart review and allows for a more streamlined approach that has been shown to be an effective and reproducible form of medication error detection [24, 25]. Many of the components that are manually evaluated in trigger tool-focused chart review can be automated by utilizing the ever-expanding technology of the EMR information systems. Automated adverse event detection or trigger tools have been developed to allow the prospective gathering of data via specific signals or triggers. Several EMR trigger tools have been implemented and reviewed in the general pediatric setting [26–28] and in the pediatric hematology and oncology subspecialty setting as well [29]. These trigger tools focus on discrete events that occur in the EMR, and reports can be created that prompt further investigation into potential medication errors. Several multi-institutional collaborations have been formed, such as the Automated Adverse Event Detection Collaborative (AAEDC), with the goal of improving the detection, collection, and analysis of medication events among groups of academic pediatric hospitals [26]. The triggers utilized by the AAEDC cover a wide range of medication and laboratory values and are summarized in Table 10.3.
Table 10.3
Triggers utilized by the Automated Adverse Event Detection Collaborative
Medication administration | Laboratory results |
|---|---|
Digoxin immune fab | Anti-Xa > 1.5 |
Flumazenil | aPTT > 100 s |
Hyaluronidase | Bilirubin > 25 mg/dL |
Sodium polystyrene | Creatinine doubling |
Naloxone | Glucose < 50 mg/dL |
Protamine | INR > 4.0 |
Acetylcysteine | Potassium > 6 mmol/L |
Glucagon | |
Vitamin K |
These triggers can be utilized in a wide variety of EMR information systems and utilized as both retrospective auditing tools and real-time interventions to prevent an error before it occurs. A more specific tool for the automated detection of medication errors in pediatric hematology oncology has been developed and evaluated at St. Jude Children’s Research Hospital (SJCRH) with a focus on supportive care and chemotherapy-related medications including protamine, vitamin K, sodium polystyrene, naloxone, flumazenil, and hyaluronidase [29]. This trigger tool noted that when one of the listed medications was ordered and administered to a patient, it was logged and the data could be extracted into a report. These triggers for potential medication errors were reviewed by both a pharmacist and physician. After review, if a medication event was truly linked to the trigger, data was collected regarding the event similar to that of an incident report. Trigger tools like the one developed at SJCRH can be developed within most existing EMR information systems and help automate the process of medication error detection. As with chart review and other forms of measurement, the data from these triggers must be then reviewed and classified in order to identify interventions to prevent future errors [26, 29].
Electronic trigger tools build upon the medication error capturing of a chart review in a quicker and automated process. This tool can allow for decreased sampling bias that can be seen with manual trigger identification via chart review. As with chart review, it allows for near real-time detection of medication errors which can help facilitate timelier investigations. Several studies have shown this tool to be a valuable addition to traditional measurement options as it has shown minimal overlap (1.9–7.8%) with voluntary incident reporting systems [26–28]. A major limitation in automated trigger tools are the lack of fully validated trigger events specifically related to chemotherapy, such as the use of methylene blue in ifosfamide neurotoxicity or timing of leucovorin administration following high-dose methotrexate infusion. Many of the validated trigger tools focus on identifying errors of supportive care medicines or hematology-related medications such as anticoagulants. As institutions continue to evaluate new chemotherapy-related triggers, it is important that these triggers proceed through an important validation phase. If the trigger tool has been validated appropriately, it can be as sensitive as chart review and more sensitive than incident report review at identifying medication errors [11].
During the validation process, it is important for hospital systems to evaluate the positive predictive value of each medication trigger. For example, during the validation study of the SJCRH trigger tool, no events were detected associated with the use of vitamin K. The study concluded that if the tool had been restricted to the use of patients only with known concurrent use of warfarin, that data may have been more helpful for review. It is therefore important that when a trigger tool is implemented, that there be a process of internal review to identify the predictive value of errors based on trigger to ensure continued measurement will be beneficial. There can be significant financial investments in the development and implementation of a trigger tool, but once implemented many studies have shown trigger tools to require the least resources to continually review medication errors [20, 30, 31]. As more tools are developed and validated, this field will continue to expand as a complimentary and potentially primary medication error measurement tool.
Direct Observation
Direct observation refers to real-time evaluation techniques throughout the medication process. This technique is one of the oldest methods of detecting medication errors and has been studied since the 1960s [32]. This measurement technique involves real-time auditing of the practices of prescribing, preparing, dispensing, administration, and monitoring of medications [11, 33]. Direct observation of nursing by review teams has been the backbone of direct observation, and over time this has been expanded to include both provider and pharmacist observation as well. Examples of observations include on the prescriber level of ensuring double provider signatures for chemotherapy ordering, on the pharmacy level by selecting correct diluents for medications, and on the nursing level of appropriate administration rates and times. Errors are noted in real time and data is collected for analysis.
This measurement tool has been considered the gold standard of identifying medication errors. A systematic review of medication safety assessment practices showed that direct observation revealed the highest number of error reports, in some studies up to 400-fold the number of reports compared with incident report review, chart review, or trigger tool [11]. As with any scientific study, there are concerns as that observer influence can be involved and significant [11, 29, 33]. The review teams often include representatives from physician, pharmacy, and nursing staffs and can be resource demanding regarding the time needed for this ongoing observation [11, 33]. Some of the limitations of other measurement techniques are avoided as knowledge of the errors by subjects is not needed and willingness to report the errors is not required. As with all other measurement techniques reviewed, once the errors have been measured, an infrastructure must be in place to review and analyze the errors.
The measurement of medication errors is key to understanding the processes involved with the error and subsequently creating system changes to ensure prevention of future errors. Many studies have evaluated the costs and benefits of the individual measurement techniques. While the reviewed methods have distinct advantages and disadvantages, no study has shown the clear advantage of one stand-alone system. It is critical to understand that a multifaceted approach to measurement will be key when a healthcare organization approaches improvement strategies for patient safety regarding chemotherapy and other medications.
Improvement Strategies
Introduction
Organizational strategies for improving chemotherapy and medication safety should focus on the overall chemotherapy process including chemotherapy prescription, preparation, and administration [1, 7, 34, 35]. Institutions must ensure that safeguards are in place at each step and should place these strategically within the chemotherapy use process. A successful system design should account for psychological precursors and human factors and incorporate standardization, technology, patient input, and double checks to ensure that errors are prevented [1, 36].
Standardization
One of the most effective ways to minimize error is to standardize the process and tools used to prescribe, dispense, and administer chemotherapy. A lack of standardization can create an environment of confusion, misinterpretation, and variability in these processes. One way to improve the chemotherapy process is to evaluate each of these steps and implement evidence-based strategies that can minimize errors [7, 34, 37].
Prescribing
It is well known that incomplete, illegible, or incorrect chemotherapy orders can lead to ambiguity and misinterpretation, thereby putting patients at significant risks [1]. Patient care facilities should utilize standardized pre-printed or electronic order sets whenever possible and at least for commonly used regimens and treatments. This tool helps to simplify the ordering process in that much of the basic information is prefilled [7, 37, 38].
Best practice recommendations suggest that the basic elements of all chemotherapy orders should include patient demographic information and treatment plan, hydration orders if applicable, supportive care medications, and chemotherapy medications. The orders should be presented in a standard format, in the order which they will be administered and incorporate general medication safety principles (Table 10.4). Specific recommendations for each component of the order template include the following [1, 7, 38–42]:
- 1.
Patient demographics:
- (a)
At least two patient identifiers, including the patient’s name
- (b)
Patient-specific dosing parameters: Height (cm), weight (kg), and BSA (m2)
- (c)
Diagnosis
- 2.
Treatment plan:
- (a)
Name of treatment regimen and protocol number for research regimens
- (b)
Treatment intent (optional)
- (c)
Current day and cycle, cycle length, and total number of anticipated cycles
- (a)
- 3.
Hydration orders:
- (a)
Solution type
- (b)
Volume of solution
- (c)
Route of administration
- (d)
Duration of infusion
- (a)
- 4.
Supportive care medications (pre-chemotherapy)
- (a)
Include default medication choices. These should be customized to the regimen given and meet evidence-based practice guidelines
- (b)
Alternative or add-on options should be available for situations in which the patient does not respond appropriately to default medication choices. These options should also be based on clinical practice guidelines
- (c)
Standardized full generic name, dose, route of administration, duration of infusion as applicable, and time of administration in relation to chemotherapy should be included.
- (a)
- 5.
Chemotherapy medications
- (a)
Standardized full generic name of the chemotherapy agent.
- (b)
Brand names should be included in situations of look-alike-sound-alike medication names.
- (c)
Dosing unit (mg/m2, mg/kg, etc.) and patient calculated dose.
- (d)
Reasons for dose modifications.
- (e)
IV solution and volume and duration of infusion as applicable.
- (f)
Drug dosages and calculated doses should be expressed according to the “container” rule (i.e., the calculated dose is the amount prepared and administered from a single container).
- (g)
Chemotherapy medications infused over multiple days (continuous infusions) should include total daily dose and total dose over total length of time (i.e., 1200 mg/m2/day IV continuous over 24 h on days 1 and 2 (2400 mg/m2 IV over 48 h).
- (h)
Solution types, volume, and duration of infusion should be standardized for each chemotherapy medication.
- (i)
The administration schedule, including frequency of administration and days on which each dose is to be given within a treatment cycle or course, should be specified.
- (a)
- 6.
Get Clinical Tree app for offline access
Supportive care medications (post chemotherapy)
- (a)
Growth factor support
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



- (a)