• Intrathecal administration
• Convection enhanced delivery
• Blood Brain Barrier disruption
• Intra-arterial administration
• Intratumoral delivery
Intrathecal administration of drugs like Ara-C and mafosfamide has been used in medulloblastoma, pineoblastoma, and recurrent brain tumors to bypass the BBB. The persistent therapeutic challenge due to nonhomogeneous dispersion is associated with ineffective volume of drug dispersion due to varying molecular weights and diffusivities. In addition, one must consider that follow-up neuropsychological evaluations suggest that these therapeutic regimens are not without consequences to the developing nervous system (Partap et al. 2011; Blaney et al. 2005).
Convection enhanced delivery (CED) is another regional administration method of biologic agents using intracranial catheters connected to target sites with a continuous positive pressure gradient. This provides improved delivery of molecules to the tumor and overcomes limitations of intrathecal chemotherapy. Using this technique, delivery of transferrin-CRM107, a conjugate of human transferrin (Tf) and a genetic mutant of diphtheria toxin (CRM107) to tumoral areas, showed peritumoral toxicity, tumor response, and safety in adults. Studies are in development to translate this application to deliver immunotoxins to tumoral cavities after surgical resection of pediatric brain tumors (Laske et al. 1997). Recently, CED delivery of topotecan has shown responses in glioblastoma and currently is in clinical trial for patients with DIPG (Bruce et al. 2011); however, challenges continue in optimal defining of convective distribution based on volume of agents infused, and concerns of retrograde reflux of drugs which can cause CNS toxicity.
BBB disruption is a method used to enhance drug delivery to tumor sites by using vasoactive substances. This allows therapeutics to enter the brain through this barrier. Bradykinin, a potent vasodilator, and its analogue lobradimil (RMP-7) have been used in clinical trials to enhance and facilitate the entry of carboplatin in treating PNET, HGG, and ependymoma. Though initial response was seen in phase I trials, the use of lobradimil and carboplatin in phase II trials was found to be inactive in childhood HGG and other tumors (Felix et al. 2013; Warren et al. 2006; Ford et al. 1998).
Intra-arterial (IA) delivery of chemotherapeutic agents through cannulated carotid or vertebral artery has been used for over three decades in different malignancies with evidence of higher concentration of infused agents at tumor sites (Hori et al. 1987). This approach has been performed alone or with the use of agents like mannitol, which induce BBB disruption, and enhances delivery of drugs. Phase III studies in adults with glioblastoma using this modality showed increased toxicities and no survival benefit over conventional delivery techniques (Imbesi et al. 2006; Hiesiger et al. 1995). Few studies using IA chemotherapy in children with brain tumors have been performed. Methotrexate or carboplatin delivered through this route in CNS tumors showed objective responses in some patients (Dahlborg et al. 1998). IA use of cisplatin or carboplatin in adults and children with brain stem glioma along with etoposide and intravenous interferon has been studied and indicates good responses in patients with reasonable tolerability (Fujiwara et al. 1994). Another study using IA chemotherapy with disruption of BBB was performed in 27 patients as a salvage therapy for recurrent CNS tumors, which showed some promising survival results and acceptable toxicity data (Jahnke et al. 2008). The use of IA melphalan in DIPG patients is in clinical trial. Though this route of administration sounds promising, to date, no data has shown benefit over intravenous route. Further studies need to be profiled to conclusively define safety and efficacy of this route of administration of biologic agents in children with brain tumors.
Intratumoral use of chemotherapeutic agents into CNS tumors directly using chemotherapy impregnated wafers, gels, spheres, or injection of agents into the site has been evaluated. In addition to overcoming the BBB, it also helps in reducing dispersion of the drug beyond the desired site. Adult studies using wafers containing BCNU within a biodegradable polymer (Gliadel®) were inserted at the surgical sites in recurrent or newly diagnosed malignant gliomas. This approach showed some efficacy; however, toxicities included cerebrospinal fluid leak, edema, and treatment-related necrosis (Nagpal 2012; Valtonen et al. 1997). To enhance clinical efficacy, use of carmustine wafers followed by chemoradiotherapy with temozolomide in adults with newly diagnosed malignant gliomas showed a modest overall survival of 18 months (Kleinberg et al. 2004). A limitation of the use of these wafers is the restricted diffusion of chemotherapeutic agents across tissues beyond implanted surgical cavity site, undermining its access and clinical efficacy in infiltrative brain tumors. Although the role of these agents has been demonstrated in adults, no study has shown convincing safety and efficacy of its use in childhood brain tumors.
5.2.6.4 Targeted Chemotherapy
Over the last two decades, great strides have been made in the discernment of the molecular biology of childhood brain tumors, enabling us to better understand the oncogenic drivers that induce angiogenesis, tumor invasion, and migration. This knowledge has led to the development of targeted therapeutic trials (Fig. 5.1, Table 5.2). Key receptor tyrosine kinases include epidermal growth factor receptor (EGFR), platelet derived growth factor receptor (PDGFR), and vascular endothelial growth factor receptor (VEGFR). Major signaling pathways involved in tumorigenesis of childhood CNS tumors are phosphatidylinositol 3-kinase/Akt/mTOR, Ras-mitogen-activated protein kinase, and the Sonic Hedgehog (SHH) (de Bont et al. 2008; Brantley and Benveniste 2008; Lo 2010; Nageswara Rao et al. 2012).
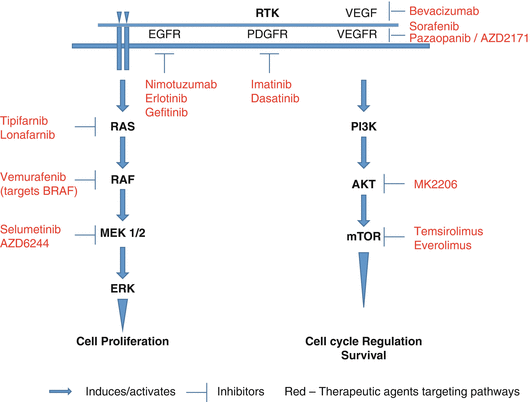

Fig. 5.1
Cell signaling pathways and therapeutic targets. AKT protein kinase B, EGFR epidermal growth factor receptor, ERK extracellular signal-regulated kinase, MEK mitogen-activated protein kinase, mTOR mammalian target of rapamycin, PDGFR platelet derived growth factor receptor, PI3K phosphatidylinositol 3-kinase, RAS Ras proteins, RTK receptor tyrosine kinase
Table 5.2
Targeted therapy in pediatric CNS tumors
Target | Drug |
|---|---|
EGFR | Nimotuzumab, erlotinib, gefitinib |
PDGFR | Imatinib, dasatinib |
PI3K/AKT/mTOR pathway | MK2206, everolimus, temsirolimus |
Ras/Raf/MEK/ERK pathway | Tipifarnib, lonafarnib, AZD6244 |
Angiogenesis/VEGF pathway | Bevacizumab, pazopanib |
Histone deacetylase | Suberoylanilide hydroxamic acid, valproic acid |
Sonic Hedgehog pathway | GDC0449, LDE225 |
BRAF mutation | Vemurafenib |
Nimotuzumb, an IgG1 antibody that targets the EGFR, has been used in pediatric HGG, which was tolerated well and yielded a 2-year survival rate of 54.2% (Cabanas et al. 2013). Erlotinib a potent and selective oral inhibitor of EGFR and HER2, used along with local radiotherapy in newly diagnosed pediatric HGG showed acceptable toxicity but did not change the poor outcome of these tumors (Qaddoumi et al. 2014). Gefitinib another oral inhibitor of EGFR when used along with radiotherapy in newly diagnosed DIPG showed tolerability and an overall survival rate of 12 months, no better than historical controls (Pollack et al. 2011). PDGFR alpha has been shown to be expressed in HGG and medulloblastoma and remains an attractive target. Imatinib is a small molecule inhibitor of the kit oncogene and PDGFR. Though it has been used in clinical trials, its poor outcome due to its limited CNS penetration has undermined therapeutic efficacy and also showed risk of intratumoral hemorrhage (Baruchel et al. 2009). Dasatinib, a potent PDGFR inhibitor with better CNS penetration, is now being developed in clinical trials (Broniscer et al. 2013).
The PI3K/Akt/mTOR pathway is well known to drive downstream signaling in various childhood brain tumors. MK2206, an allosteric inhibitor of Akt, is currently in clinical trials in recurrent/refractory gliomas, based on its efficacy in malignant gliomas, when used with other synergistic therapies (Cheng et al. 2012). mTOR is a downstream major gatekeeper receiving signals from Ras/Raf/MAPK/ERK and the PI3K/Akt/mTOR pathway, inducing oncogenic events. Drugs like everolimus and temsirolimus which inhibit this key mTOR molecule are being evaluated in clinical trials based on preliminary studies demonstrating efficacy in brain tumors (Cappellano et al. 2013; Piha-Paul et al. 2014).
Ras is an effector molecule in the Ras-mitogen-activated protein kinase pathway. Activation of growth factor receptors leads to farnesylation of the Ras molecule by farnesyl transferase (FT), which induces its activation. Blocking this phenomenon using FT inhibitors tipifarnib and lonafarnib has been studied. Though these drugs were well tolerated, no clinical benefit was seen as monotherapy in recurrent CNS tumors thus far (Haas-Kogan et al. 2011; Kieran et al. 2007). Selumetinib (AZD6244), a mitogen-activated protein kinase inhibitor against MEK1/2, showed activity against juvenile pilocytic astrocytoma xenografts in preclinical testing (Kolb et al. 2010). Based on this data, the PBTC is performing a clinical trial using AZD6244 in recurrent refractory LGG (ClinicalTrials.gov Identifiers NCT01386450 and NCT01089101). The BRAF V600E mutation seen in pediatric gliomas results in constitutive activation of the BRAF kinase with subsequent activation of the MAPK/ERK pathway (Schindler et al. 2011). To date, few reports of response to pediatric LGG and HGG to vemurafenib, a BRAF inhibitor, have been published (Skrypek et al. 2014; Robinson et al. 2014).
Angiogenic inhibitors targeting the VEGF are now well established. Bevacizumab, a humanized monoclonal antibody that is highly specific for all VEGF isoforms, is now being used in recurrent pediatric brain and optic pathway tumors with disease response and variable toxicities (Gururangan et al. 2014; Avery et al. 2014). Small molecule inhibitors of the VEGF pathway, pazopanib and AZD2171, along with multikinase inhibitor (MTKI), sorafenib, that has angiogenic properties are in various phase I/II pediatric clinical trials.
Activation of the Sonic Hedgehog (SHH) pathway is identified in nearly 25% of medulloblastoma. SHH ligand binding to transmembrane protein PTCH leads to disinhibitory effects of the PTCH gene. This results in constitutive activation of the transmembrane protein SMO, which in turn activates downstream glioma associated oncogene homolog 1 proteins, resulting in tumorigenesis. Preclinical data and clinical responses in patients led to the translation of specific SMO inhibitors vismodegib (GDC-0449) and LDE225 into clinical trials. Preliminary data showed that these drugs were well tolerated with some antitumor activity (Gajjar et al. 2010; Geoerger et al. 2012).
With the emerging era of epigenetics in brain tumors, the role of histone acetylation and deacetylation (HDAC) in chromatin remodeling and oncogenesis is gaining importance. HDAC inhibitors, vorinostat and valproic acid, are associated with variable responses in recurrent CNS tumors (Hummel et al. 2013; Felix et al. 2013). Valproic acid was well tolerated in heavily pretreated pediatric patients with HGG (Wolff et al. 2008).
Though explosion in the data from the molecular and genomic research on childhood brain tumors continues to temper enthusiasm, challenges remain in keeping pace with designing optimal trials with meaningful therapeutic benefit against these formidable malignancies.
References
Adamson P, Balis FM, Berg SL (2006) General principles of chemotherapy. In: Pizzo PA, Poplack DG (eds) Principles and practice of pediatric oncology. Lippincot, Williams and Wilkins, Philadelphia
Agarwal S, Mittapalli RK, Zellmer DM, Gallardo JL, Donelson R, Seiler C, Decker SA, Santacruz KS, Pokorny JL, Sarkaria JN, Elmquist WF, Ohlfest JR (2012) Active efflux of Dasatinib from the brain limits efficacy against murine glioblastoma: broad implications for the clinical use of molecularly targeted agents. Mol Cancer Ther 11(10):2183–2192. doi:10.1158/1535-7163.MCT-12-0552 CrossrefPubMedPubMedCentral
Ater JL, Zhou T, Holmes E, Mazewski CM, Booth TN, Freyer DR, Lazarus KH, Packer RJ, Prados M, Sposto R, Vezina G, Wisoff JH, Pollack IF (2012) Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J Clin Oncol 30(21):2641–2647. doi:10.1200/JCO.2011.36.6054 CrossrefPubMedPubMedCentral
Avery RA, Hwang EI, Jakacki RI, Packer RJ (2014) Marked recovery of vision in children with optic pathway gliomas treated with bevacizumab. JAMA Ophthalmol 132(1):111–114. doi:10.1001/jamaophthalmol.2013.5819 CrossrefPubMed
Baruchel S, Sharp JR, Bartels U, Hukin J, Odame I, Portwine C, Strother D, Fryer C, Halton J, Egorin MJ, Reis RM, Martinho O, Stempak D, Hawkins C, Gammon J, Bouffet E (2009) A Canadian paediatric brain tumour consortium (CPBTC) phase II molecularly targeted study of imatinib in recurrent and refractory paediatric central nervous system tumours. Eur J Cancer 45(13):2352–2359. doi:10.1016/j.ejca.2009.05.008 CrossrefPubMed
Blaney SM, Boyett J, Friedman H, Gajjar A, Geyer R, Horowtiz M, Hunt D, Kieran M, Kun L, Packer R, Phillips P, Pollack IF, Prados M, Heideman R (2005) Phase I clinical trial of mafosfamide in infants and children aged 3 years or younger with newly diagnosed embryonal tumors: a Pediatric Brain Tumor Consortium study (PBTC-001). J Clin Oncol 23(3):525–531. doi:10.1200/JCO.2005.06.544 CrossrefPubMed
Bouffet E, Khelfaoui F, Philip I, Biron P, Brunat-Mentigny M, Philip T (1997) High-dose carmustine for high-grade gliomas in childhood. Cancer Chemother Pharmacol 39(4):376–379. doi:10.1007/s002800050586 CrossrefPubMed
Bouffet E, Jakacki R, Goldman S, Hargrave D, Hawkins C, Shroff M, Hukin J, Bartels U, Foreman N, Kellie S, Hilden J, Etzl M, Wilson B, Stephens D, Tabori U, Baruchel S (2012) Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol 30(12):1358–1363. doi:10.1200/JCO.2011.34.5843 CrossrefPubMed
Brantley EC, Benveniste EN (2008) Signal transducer and activator of transcription-3: a molecular hub for signaling pathways in gliomas. Mol Cancer Res 6(5):675–684. doi:10.1158/1541-7786.MCR-07-2180 CrossrefPubMed
Broniscer A, Baker SD, Wetmore C, Pai Panandiker AS, Huang J, Davidoff AM, Onar-Thomas A, Panetta JC, Chin TK, Merchant TE, Baker JN, Kaste SC, Gajjar A, Stewart CF (2013) Phase I trial, pharmacokinetics, and pharmacodynamics of vandetanib and dasatinib in children with newly diagnosed diffuse intrinsic pontine glioma. Clin Cancer Res 19(11):3050–3058. doi:10.1158/1078-0432.CCR-13-0306 CrossrefPubMedPubMedCentral
Bruce JN, Fine RL, Canoll P, Yun J, Kennedy BC, Rosenfeld SS, Sands SA, Surapaneni K, Lai R, Yanes CL, Bagiella E, DeLaPaz RL (2011) Regression of recurrent malignant gliomas with convection-enhanced delivery of topotecan. Neurosurgery 69(6):1272–1279. ; discussion 1279–1280. doi:10.1227/NEU.0b013e3182233e24 CrossrefPubMedPubMedCentral
Cabanas R, Saurez G, Rios M, Alert J, Reyes A, Valdes J, Gonzalez MC, Pedrayes JL, Avila M, Herrera R, Infante M, Echevarria E, Moreno M, Luaces PL, Ramos TC (2013) Treatment of children with high grade glioma with nimotuzumab: a 5-year institutional experience. mAbs 5(2):202–207. doi:10.4161/mabs.22970 CrossrefPubMedPubMedCentral
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree