Model
Lung unilateral embolization with DSM ± carboDDP, rats: study of pulmonary microcirculation by measurement of FITC-labeled erythrocytes
N
12 (2 × 6)
Objective
Pulmonary microcirculation
Comparisons
1. unilateral embolization with DSM
2. unilateral embolization with DSM + carboDDP
Embolization
30 mg/kg amilomer (DSM)
Results
Mean flow retardation: 14 min
Original flow of erythrocytes: 21 min after embolization (Reperfusion and reversibility of microembolization)
Confirmation of patency of the central pulmonary artery by pulmonary angiogram
No cause of pulmonary edema through the additional application of carboplatin
Conclusions
For the first time unilateral microembolization of the lung could be established in an experimental model. By injection of DSM, reversible embolization on arteriolar and capillary level could be demonstrated without occlusion of the main branches of the pulmonary arteries. Alveolar-capillary membrane disorder as symptom of early toxicity could not be detected even with additional application of carboplatin
Schneider et al. (2002) [34]
Tumor model | Lung tumor model (adenocarcinoma), rats |
N | 25 (5 × 5) |
Objective | Tumor control in lung metastases |
Comparisons | 1. ILP with buffered starch solution |
2. DSM mono | |
3. CarboDDP I.V. | |
4. ILP with carboDDP | |
5. DSM + carboDDP | |
Embolization | Amilomer (DSM) |
Results | Tumor volumes after 7 days after therapy (size differences): |
1. 422 mm3 | |
2. 697 mm3 | |
3. 70 mm3 | |
4. −8 mm3 | |
5. −17 mm3 | |
3 vs. 4 + 5 p < 0.005 | |
Conclusions | This is the first study to perform chemoembolization of the lung. Compared with i.v. therapy, chemoembolization was more effective without serious toxicity. Its efficacy was comparable with that of isolated lung perfusion but less stressful for a possible clinical application |
Pohlen et al. (2007) [40]
Model | TACE of lung tumor model (adenocarcinoma), rats |
N | 60 (3 groups of 5 animals each and 4 times of measurement (15, 30, 60, and 120 min) |
Objective | Pharmacokinetics, histology of tumor tissue |
Method | 1. 45 mg/kg carboDDP I.V. |
2. ILP (15 mg/kg carboDDP) | |
3. TACE (2 mg/kg DSM + 15 mg/kg carbDDP) | |
TACE | 2 mg/kg DSM + 15 mg/kg carboDDP |
Results | PK: 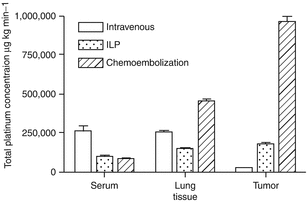 |
Histology: | |
No fibrotic changes detected in any group. ILP and TACE group showed evidence of mild alveolar cell hyperplasia and pulmonary edema | |
Conclusions | This is the first study to measure the concentration of carboplatin during chemoembolization of the lung. Compared to intravenous therapy, chemoembolization produced higher tumor tissue concentrations. Comparing chemoembolization to ILP, there was also an increase of carboplatin in the tumor tissue, without histological damage of the surrounding lung parenchyma |
Pohlen et al. (2007) [41]
Model | TACE of lung, pig |
N | 6 |
Objective | Safety and effectiveness of this method in a large animal model |
Method | Puncture of femoral vein, selective exploration of the tumor-supplying pulmonary arteries, chemoembolization with DSM and carboplatin, documentation of survival, hemodynamic parameters, ventilation gas exchange, digital subtraction angiography (DSA), and pulmonary X-rays during and after chemoembolization |
TACE | 1–2 mg/kg DSM + 15 mg/kg carboDDP |
Results | All the animals survived the operative procedure and chemoembolization. None of the animals showed clinical disturbances in the period between chemoembolization and sacrifice 6 months later. Body weight showed an increase |
Conclusions | This is the first study of chemoembolization of the lung in a large animal model. The feasibility, mild hemodynamic acute effects and the absence of long-term toxicity were documented. These observations justify patient studies in unresectable lung tumors |
van Putte et al. (2008) [42]
Concept | Isolated lung perfusion with gemcitabine in pigs (catheterization model of selective pulmonary artery perfusion (SPAP) combining the properties of isolated lung perfusion) |
N | 20 |
Procedure | Five groups (N = 4, each) gemcitabine in a dose of 1 g/m2 : |
SPAP with a normal pulmonary artery blood flow for 10 min | |
SPAP with a normal pulmonary artery blood flow for 2 min | |
Control (IV) | |
SPAP for 2 min with 50 % | |
SPAP for 2 min with 90 % flow reduction within the pulmonary artery | |
Results | The peak concentration of gemcitabine within the serum was significantly higher after SPAP for 2 min compared with i.v. infusion (p = 0.004) |
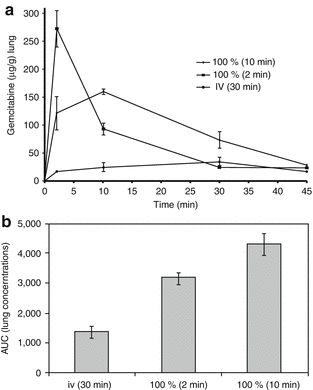 | |
Flow reduction during SPAP for 50 and 90 % did not result in a significantly different lung and serum AUC compared with SPAP without flow reduction | |
Toxicity | Histological examination: evidence of slight alveolar hyperplasia (more pronounced in the flow reduction groups with evident moderate congestion). No alveolar hyperplasia in the i.v. group. No abnormalities in the slight sections of the pulmonary artery in either the SPAP or the i.v. group |
Conclusions | We advocate SPAP as a new method to be tested clinically to achieve downstaging of the tumor and lymph node status in lung cancer |
8.2.2 Clinical Data
8.2.2.1 Practicability
Please note:
Inclusion criteria: relapsed liver metastases after partial liver resection, metastases in both liver sides, unresectable foci, general contraindications for operation, patients’ decision, ≤5 lesions with ≤5 cm size per metastasis
Safety parameter for patients for sequential LITT
Treatment phase | Action |
|---|---|
Before treatment | Hepatitis, fever, blood count, clotting (e.g., Hk, PTT, part. TPT) |
Intraprocedural | Clinical investigations: |
Pulse, blood pressure, blood oxygen | |
Medication: | |
Local anesthesia (1 % mepivacaine) | |
Sedation (diazepam) | |
Antibiotics (2 g cefotiam) | |
Analgesia (opiates, e.g., piritramide and pethidine i.v.) | |
Postprocerdural (immediately) | Clinical investigations: |
Pulse, blood pressure (every 30 min over 6 h) | |
Medication: | |
Analgesia (opiates, e.g., piritramide and pethidine i.v.) | |
Antinausea (e.g., metoclopramide) | |
Hydration | |
After 10 days | Hepatitis, fever, breathing frequency |
8.2.2.2 Study Results
Isolated Lung Perfusion (ILP)
Schröder et al. (2002) [43]
Concept | Isolated lung perfusion with high-dose chemotherapy for the treatment of surgically relapsing or unresectable lung sarcoma metastasis |
N | 4 |
Inclusion criteria | Unilateral or bilateral sarcoma metastasis confined to a lobe or entire lung, drug-resistant metastasis and at least four previous surgical metastasectomies |
Therapy | For 20–40 min at a rate of 0.3–0.5 l/min, a mean perfusion pressure lower than the own mean pulmonary artery pressure (inflow temperature: 41 °C or higher) |
Results | Median follow-up: 12 months |
N = 3: alive and disease-free (N = 1 death from cerebral metastasis without autopsy evidence of local recurrence 13 months following ILP) | |
Toxicity | No systemic drug-related toxicity, all patients experienced transient pulmonary toxicity as noncardiogenic edema of the treated lung segments |
Conclusions | Hyperthermic perfusion chemotherapy can be done safely and effectively. It represents a new treatment modality and deserves further investigations for patients with advanced, drug resistant, or surgically refractory, lung sarcoma metastasis |
Hendriks et al. (2006) [9]
Concept | Isolated lung perfusion with melphalan for resectable lung metastases – phase I |
N | 16 |
Inclusion criteria | Resectable pulmonary metastases only |
Therapy
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|