Fig. 6.1
Regulation of the developmental program of satellite cells. (a) Schematic illustrating the myogenic lineage progression. Satellite stem cells are marked by the expression of Pax7, but have never expressed Myf5 in their developmental history. Satellite stem cells maintain the satellite cell pool by generating satellite cells expressing Myf5 while also undergoing self-renewal. Upon muscle injury satellite cells become myoblasts through the upregulation of MyoD. Once differentiation is initiated Pax7 is downregulated and Myogenin and MRF4 are upregulated. This leads to the conversion of myoblasts into fusion competent myocytes. Subsequently myocytes will fuse to form syncitial myotubes which start to express genes coding for sarcomeric proteins such as MHC. These myotubes will then mature into fully developed and innervated fibers that are marked by proteins typical for the respective fiber type (e.g. MHC fast or slow). (b) Fluorescence microscopy images representative of myogenic lineage progression demonstrating morphological changes during linage progression
Adult skeletal muscle contains different muscle fibers with different fiber type compositions that can be generally categorized into slow and fast contracting ones. Diversification of muscle fibers into distinct types is observed early in development with the initial phase being independent of neural input. In mice, all muscle fibers express embryonic MHC and slow MHCI before ED16. Some of these fibers lose expression of slow MHCI and acquire expression of neonatal MHC. Fast and slow fibers arise from these two populations of primary fibers. Concomitant with this process secondary fibers are generated which either express embryonic MHC or neonatal MHC but no slow MHC. In mice, skeletal muscle is still immature at birth and the fiber type profile changes further during postnatal development. Subsequently, embryonic and neonatal MHC expression is mostly replaced by MHC2A (fast oxidative). This is followed by the upregulation of all fast isoform MHC genes leading to the expression of MHC2A (fast oxidative), fast MHC2X (fast intermediate) and MHC2B (fast glycolytic) in specific fiber subpopulations. In fast contracting murine muscles, such as the tibialis anterior muscle, slow MHCI positive fibers disappear while in slow contracting muscles such as the soleus MHC2A positive fibers transform into slow MHCI expressing fibers. These changes in perinatal fiber composition are critically controlled by the maturation of neuromuscular junctions, mechanical load imposed on neonatal limb muscles and changes in the level of thyroid hormone (Schiaffino and Reggiani 2011). Skeletal muscle has a very high capacity to adapt to physiological demands, e.g. increased exercise. Fiber type composition and volume of the muscle can be influenced by exercise. Hypertrophy of skeletal muscle is characterized by an increase in the size of muscle fibers. This form of adaptation involves satellite cells which under physiological conditions fuse to growing muscle fibers to maintain the size of the myonuclear domain. Moreover, the amount of satellite cells differs between muscle groups. Generally slow contracting muscles contain more satellite cells.
Satellite Cells Are a Heterogeneous Population
The satellite cell population is heterogeneous based on the expression of cell surface markers and expression levels of Myf5. It has been shown that around a fifth of satellite cells display slower proliferation kinetics and are resistant to differentiation. A recent study described the use of a Pax7-GFP live reporter which suggested that satellite cells are heterogeneous based on the expression level of Pax7 (Rocheteau et al. 2012). The authors proposed that Pax7 high expressing satellite cells are more undifferentiated than Pax7 low expressing cells and can distribute their chromatids asymmetrically.
Genetic analysis using the Cre-LoxP system (R26R-YFP/Myf5-Cre) found that approximately 10% of Pax7 positive satellite cells have never expressed Myf5 demonstrated by the absence of yellow fluorescent protein (YFP) expression. However, Kuang et al. (2007) observed up-regulation of Myf5 and induction of YFP expression in satellite daughter cells. This study revealed that Pax7+/YFP– satellite cells represent a rare stem cell population within the satellite cell pool, while Pax7+/YFP+ satellite cells, which make up the majority of satellite cells, are committed myogenic progenitors. During muscle regeneration, both Pax7+/YFP+ as well as Pax7+/YFP– satellite cells proliferate and undergo planar divisions generating two identical daughter cells that both remain either Pax7+/YFP+ or Pax7+/YFP– (Fig. 6.2). Strikingly, apical-basal divisions, with the polarity of cell division at right angles to the basal lamina, were typically asymmetrical, with the basal cell remaining YFP– and the apical cell up-regulating YFP.
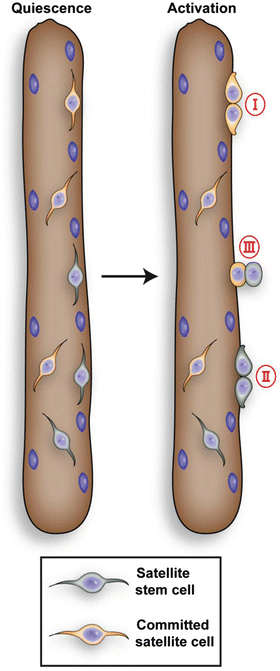

Fig. 6.2
The satellite cell population is heterogeneous. Upon activation satellite stem cells and committed progenitor cells can undergo symmetric divisions (I and II) with the orientation of the division parallel to the muscle fiber (planar). Satellite stem cells can also undergo asymmetric divisions (III) with the division perpendicular to the muscle fiber (apical-basal). This division gives rise to a committed daughter cell and a self-renewing satellite stem cell. Satellite stem cell, which never expressed myf5, comprise about 10% of total satellite cells
The Pax7+/YFP– satellite stem cells presumably represent a lineage continuum of the embryonic Pax3/Pax7 positive cells which did not express any of the MRFs (myogenic regulatory factor) (Fig. 6.2; Kuang et al. 2007). Activated Pax7+/YFP+ satellite cells then upregulate MyoD and start proliferating thereby generating myoblasts (Kuang et al. 2008). Expression of MyoD leads to the upregulation of myogenin, a downstream transcription factor responsible for the transcription of genes involved in terminal differentiation (Bentzinger et al. 2012). Recently Le Grand et al. (2009) showed that Wnt7a drives the symmetric expansion of satellite cells through the planar cell polarity pathway. Wnt7a induces the polarized distribution of the planar cell polarity effector Vangl2, thereby controlling the homeostatic level of satellite stem cells as well as the regenerative potential of the muscle. Another signaling pathway that has been implicated in the control of satellite cell numbers is the Notch signaling pathway. Repression of Notch signaling results in a reduced tissue content of satellite cells due to inhibition of satellite stem cell renewal (Conboy and Rando 2002). The fine regulation of satellite cell self-renewal is essential for maintaining the integrity of the skeletal muscle. Stimulation of the number of symmetric satellite stem cell divisions through application of specific drugs or biologics could potentially increase the regenerative capacity of skeletal muscle and might well be a potential treatment for muscle wasting diseases such as Duchenne muscular dystrophy.
Lineage Progression During Adult Myogenesis
The number of satellite cells decreases after birth. In newborn mice, satellite cells account for 30% of sublaminar muscle nuclei, this is followed by a decrease to less than 5% in 2-month old animals (Wang and Rudnicki 2012). Although the number of satellite cells is decreased in aged muscle, their intrinsic myogenic potential and self-renewal capacity remains unaltered (Day et al. 2010). Muscle grafts from old mice into a young host resulted in a robust activation and regeneration of grafted cells, while satellite cells from young mice performed poorly when transplanted into an old host suggesting that the satellite cell niche is substantially different between old and young mice (Kuang et al. 2008).
The mechanisms regulating satellite cell-lineage progression in the adult share a striking similarity with embryonic myogenesis (Fig. 6.1). It has been observed that during satellite cell activation in the adult a subset of Pax7+/MyoD+ myoblasts downregulate MyoD, withdraw from the cell cycle and return to quiescence. This subset comprises the, so called, reserve cells. Pax7 expression induces proliferation of myoblasts and delays their differentiation through regulation of MyoD expression (Olguin et al. 2007). Pax7 expression itself is affected by expression of myogenin leading to its downregulation through negative feedback during differentiation (Olguin et al. 2007). Recent analyses revealed that Pax7 not only regulates MyoD expression but also Myf5 expression in satellite cell derived myoblasts by binding to a region upstream of the Myf5 gene (Bentzinger et al. 2012).
The transcription factors MyoD and Myf5, which are both expressed after activation of satellite cells, seem to have specific roles in adult myogenesis. MyoD is required for the predetermination of myoblasts to differentiate, while Myf5 regulates their proliferation and homeostasis (Wang and Rudnicki 2012). Despite the fact that MyoD and Myf5 can compensate for their loss during embryogenesis they cannot compensate for the loss of each other during adult muscle regeneration.
The expression of myogenin and MRF4 follows the one of Myf5 and MyoD. Expression of myogenin and MRF4 is necessary and sufficient for the formation of myotubes and myofibers (Bentzinger et al. 2012). Neither myogenin nor MRF4 are involved in satellite development or satellite cell maintenance.
Satellite Cells Are Indispensable for Muscle Regeneration
Several experiments illustrated the ability of satellite cells to contribute to regeneration of skeletal muscle. Recent experiments using diphtheria toxin based ablation of satellite cells now clearly demonstrate their necessity for regeneration. When Pax7 positive cells were ablated in two different mouse models (Pax7DTR/+ and Pax7+/CE; R26ReGFP-DTA/lacZ), skeletal muscle failed to regenerate (Lepper et al. 2011; Sambasivan et al. 2011). Instead of nascent myofibers the researchers found adipose tissue infiltrating the damaged muscles. This phenotype is reminiscent of the phenotype of constitutively Pax7 deficient mice (Seale et al. 2000). These experiments suggest that satellite cells are the only contributors to regeneration of skeletal muscle. Since skeletal muscle has a very low turnover rate, satellite cells are mostly quiescent in resting muscle. However, stimuli such as injury or exercise can activate satellite cells. Hence, mice with ablated satellite cells do not display an overt phenotype unless challenged by injury.
Requirement of Pax7 for Satellite Cell Function
Pax7 plays a crucial role in satellite cell function as demonstrated by the phenotype of Pax7 deficient mice (Kuang et al. 2006; Seale et al. 2000). Pax7 deficient mice display a severe phenotype harbouring a depletion of the satellite cell pool in the early postnatal period, most animals die during the first weeks of life (Seale et al. 2000). Muscles from these mice are not able to regenerate following injury demonstrating that Pax7 expression is essential for satellite cell homeostasis in juvenile mice (Kuang et al. 2006; Seale et al. 2000). If Pax7 is inactivated in the adult muscle using inducible Cre-mice combined with a floxed Pax7 allele then mutant satellite cells have been shown to be uncompromised in the regenerating muscle (Lepper et al. 2009). These experiments suggest that satellite cells in juvenile muscles depend on Pax7 but do not require this factor in mature adult muscle. On the contrary, a recent publication suggests that Pax7 is required for regulation of expansion and differentiation of satellite cells not only during neonatal but also adult myogenesis (von Maltzahn et al. 2013).
The Satellite Cell and Its Niche
The satellite cell “niche” is a term used to describe the local microenvironment of satellite cells in muscle. This microenvironment is responsible for the identity and function of the satellite cell as a stem cell. Satellite cells are located underneath the basal lamina and are in contact with myofibers, innervating motor neurons, inflammatory cells and the microvasculature. Important components of the satellite cell niche are the host muscle fiber that transmits mechanical and chemical signals to the satellite cell, the basal lamina and the microvasculature. The basal lamina consists of the ECM (extracelluar matrix) containing laminins, collagen and proteoglycans.
Evidence for the importance of the microenvironment for satellite cell function arises from studies transplanting satellite cells on myofibers (Hall et al. 2010) and transplantations of satellite cells from old mice into young hosts as well as parabiosis experiments (Kuang et al. 2008). The transplantation of single fibers with their containing satellite cells is much more efficient than the transplantation of isolated cells. Moreover, transplantation of satellite cells from old donors into young recipients resulted in a remarkable myogenic capacity of the transplanted cells. However, when satellite cells from young donors were transplanted into old recipients the transplantation success was low. Through parabiosis experiments it was demonstrated that exposure of old mice to serum from young mice increased the regenerative capacity of ‘old’ satellite cells. These experiments demonstrate that satellite cells are strongly influenced by their microenvironment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree