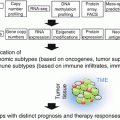
Fig. 37.1
Overview
37.1 The Immune System in the Colon and in Colorectal Cancer
The delicate balance between innate and adaptive immune system is of great importance in areas, where there is a continuous challenge of the immune system. This especially holds true for the intestinal tract. As an organ of food intake and resorption, there is a continuous influx of bacteria, viruses, foreign materials of all kinds, and noxious substances. This constant challenge of the immune system requires a finely regulated protection system, in particular on immunological level. Within such a fine tuned and balanced microenvironment, the occurrence of an intestinal tumor is a disaster on various levels. Both the innate and the adaptive immune system can recognize tumor cells as “nonself” and seek out to destroy these. So beyond protection against pathogens, this task is therefore of fundamental importance for our organism, as the detection of malignant cells—or cells that are transformed—is essential for the conservation of the cooperative integrity of the organism. In the event of tumor growth, this function is disturbed and the immune system is not able to fully contain the outgrowth of the tumor. In case of the intestine, the distinction between “nonself” and “self” is a highly complex challenge. The intestine as a niche for a huge number of bacteria that are necessary for the homeostasis of the human organism is essential. This leads to the obvious task of the intestinal immune system to keep this microcosm in balance without going to the extreme of an endless process of inflammation or—on the other side of the spectrum—infections. Inflammation is a well-known factor contributing to the neoplastic process. Chronic inflammatory bowel disease induces fundamental alterations in the complex balance in the local microenvironment. This is also reflected in the bacterial species that can be found within the intestines. The occurrence of specific invasive bacterial species [1] or the loss of protective species [2] is frequently observed. This is coupled with a significant dysregulation of the local immune system [3, 4]. It is striking that the resulting inflammatory microenvironment can be ameliorated by stool transplantation [5]. In the event of sustained inflammation, it is not unexpected to see a higher rate of tumor incidence. The precise mechanisms behind the maintenance of the delicate balance and the complexity of specific changes that lead to tumor promotion are hidden behind a complex network of (immune) cells and signaling molecules. Part of these complex networks are, e.g., dietary and lifestyle factors as well as regulatory cytokine networks within the mucosa. On the basis of large epidemiological and scientific studies, evidence suggests that the risk of colorectal cancer is increased by processed and unprocessed meat consumption but suppressed by diets rich in fiber. Moreover food composition affects colonic health and cancer risk via its modulation of colonic microbial metabolism. Gut microbiota can ferment complex dietary residues that are resistant to digestion by enteric enzymes. In this process, the release of short-chain fatty acids (including butyrate) is utilized for the metabolic needs of the colon and the body in its entirety. On a more detailed level, butyrate has a distinct effect of colonic health-promoting and antineoplastic properties. It can promote maintained mucosal integrity, and it suppresses inflammation and carcinogenesis through effects on immunity [6], gene expression, and epigenetic modulation. Only with new integrative analyses do we begin to understand the multitude of interlocking networks [7, 8]. For example, we now begin to understand, why increased iron intake can lead to tumor promotion. But beyond the parameters that influence malignant transformation and tumor initiation, the question of the role of the immune system in the tumor progression and metastatic cascade has many facets.
37.2 Immune Cells in Metastatic Colorectal Cancer: Factors for Prognosis and Therapy
This especially relates from an immunological view the invasion of tumor cells in surrounding tissues, lymph nodes and other organs. In these steps there are numerous interrogations of cellular immunologic interactions between tumor cells and the specific immune cells resident in the respective tissues. The precise interactions and the signals involved are still poorly understood. While passing through radically different immunological microenvironments, tumor cells on their way from the tumor can invade the peri-colic fat, pass through the lymph vessels, travel the peripheral blood, and spread to organs like the liver and the lungs. Starting with the primary tumor (see Fig. 37.2), one of the important immunological questions is: what immune cells are implicated in colorectal cancer? The most prominent subgroup of immune cells in colorectal carcinoma are lymphocytes. One of the first descriptions related to immunological processes in colorectal cancer can be attributed to pathologists observing the presence of lymphoid structures in the vicinity of colorectal cancer primary tumors [9]. This finding was observed in addition to the known presence of Peyer’s plaques (and other lymphoid structures) in the intestine. Only with the availability of specific staining procedures to better characterize the immunological infiltrate, pathologists, and immunologists began to systematically investigate the presence of immune cells in and around colorectal cancer [10, 11]. Not only was the presence of effector T cells confirmed by multiple groups but also the association between the composition of the local microenvironment and the clinical course of the disease across all stages of the primary tumor [11, 12]. Even for the adjuvant situation in stage III colorectal cancer, the relation of T cell density and improved overall survival was shown [13]. The analyses of the primary colorectal tumor showed especially the prognostic role of effector T cell quantities, as characterized by CD3 and CD8 surface markers. These markers, together with granzyme B, have been shown to be of relevance by independent groups and also in other cancer entities [14–22].
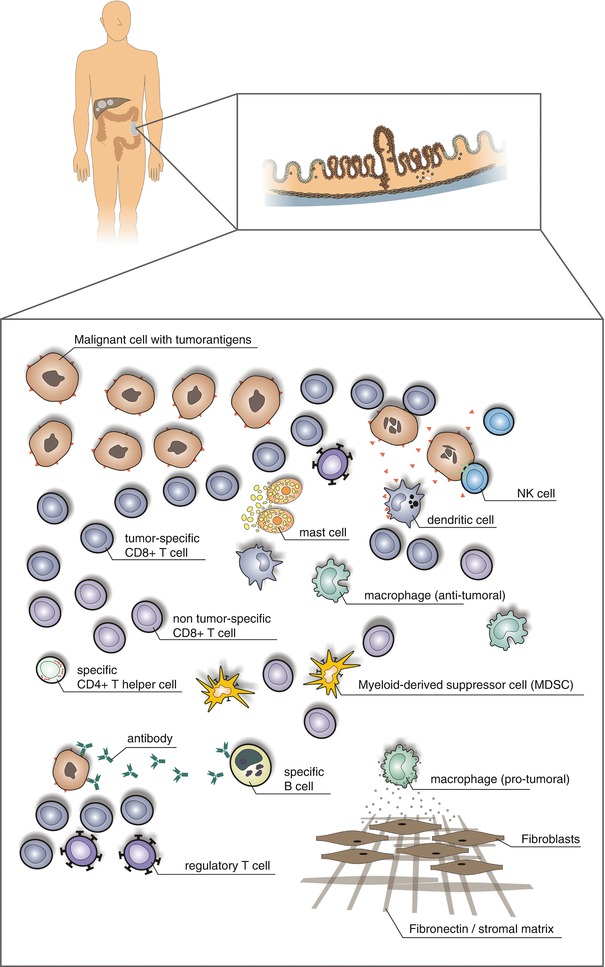

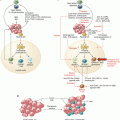
Fig. 37.2
The immunological context of the local microenvironment (from patient to microenvironment and back)
37.3 Measuring the Microenvironment: Quantification of Immune Cells in Tissues
So with the step from evaluation of lymphocytic infiltration on H&E sections to automated quantification of specifically stained lymphocyte subsets, the question of the selection of the best markers appeared. This paragraph discusses the general aspects of immune cell quantification as well as two examples that highlight ambiguities and uncertainties in this field. Classical surface markers of effector T cells are CD3, CD4, and CD8, CD3 as a pan-T cell marker and CD4 as a marker for the T-helper cells. Yet there is another T cell marker that has produced conflicting results: FOXP3. This nuclear transcription factor is found mainly in regulatory T cells, a subset of lymphocytic T cells that have inhibitory functions. Initially it was believed that their presence indicates an unfavorable microenvironment with an abrogated immune response. However, the systematic analyses in colorectal cancer revealed a different picture: they showed a positive prognostic relevance not only for CD3, CD8, granzyme B, and CD45R0 positive cells but also for FOXP3-positive lymphocytes [23]. This indicates, that in contrast to the assumptions, a higher density of FOXP3-positive lymphocytes is related to a better prognosis [24]. The explanation for this phenomenon has two components that have been clarified in the meantime. First, the immune response needs a certain level of focusing, a focusing that is driven by regulatory T cells. So a loss of regulatory and focusing immune cells leads to an abrogation of an effective immune response [25]. Second, the further analysis of FOXP3-positive lymphocyte populations revealed relevant functionally diverse subsets. So the use of FOXP3 to identify a functionally inhibitory and for the patient detrimental lymphocyte subset is not specific enough [26, 27]. In addition to the questions around ideal surface markers for quantification, the spatial distribution of these immune cells is another important parameter. The localization of immune cells is not random within the tumor tissue, but the vast majority of lymphocytes is localized within the stromal compartment [15]. Only a minor fraction of these lymphocytes is in direct contact with the tumor epithelium. One of the implications of this observation is that an antitumor effect probably is induced by the cytokines of T cells [28]. Recent data however shows that with a high-resolution analysis of the localization of T cells, the T cells directly in contact with tumor cells are the largest contributors to the prognostic benefit of increased T cell densities [29]. Looking at lymphocytes at the level of the tissue microenvironment, another important group of lymphocytes is natural killer (NK) cells. These innate immune cells with their excellent tumor killing properties were thought to have also an important role in the protection against tumor progression. NK cells have, in contrast to the T cells, a decisive advantage: tumor cell killing is antigen independent. NK cells are capable of activation if inactivating ligands are not present on the surface of target cells [30]. Particularly in the case of colorectal cancer cells, the destruction of tumor cells with loss of HLA class I molecules by NK cells has been very effective in in vitro experiments. Therefore the hypothesis was for NK cells to be contributors to a good prognosis in tumor tissue. Systematic analyses of colorectal cancer specimens with specific identification of localization and density of NK cells within the tissue revealed a different picture [31]. Already at a very early stage, in adenomas the numbers of NK cells in the tumor are massively decreased. This stands in stark contrast to the presence of chemokines and adhesion molecules that would support the presence and activation of NK cells. This surprising observation was found in all stages from adenoma to colorectal cancer liver metastases. Similar data could also be obtained from breast cancer [32–34]. Another group of lymphocytes that has not been openly implicated in tumor progression in colorectal cancers: B cells. However, antibody responses against tumor-associated antigens have been reported also in colorectal cancer patients [35–40]. B cells and plasma cells are typically found in the microenvironment, and systematic analysis has identified a possible beneficial role [41–43]. It remains to be seen whether and how these cells influence tumor growth.
37.4 Immune Cells in Metastatic Colorectal Cancer: Prognostic and Predictive Implications
With the comparison of lymphocytes across different stages of colorectal cancer, the metastatic stage is tightly linked to chemotherapy and salivatory resection of liver metastases. Prognosis is dire for patients with irresectable metastatic colorectal cancer, and survival rates are showing median 28 months [44]. Again for resected metastases, the quantity of T cells was prognostic for the clinical course after resection [45]. Chemotherapy is the only therapeutic option for patients with irresectable disease. Whole slide imaging quantification of colorectal cancer liver metastases revealed the association between effector T cell density specifically at the invasive margin and response to chemotherapy [46]. This finding was later independently validated by another research team [47]. An interesting aspect of these observations is that this relationship is not limited to irinotecan or oxaliplatinum-based chemotherapy and is not influenced by concomitant antibody therapy. While infiltrate densities could predict chemotherapy responses, from the clinical perspective the robustness of cell infiltration within the tissue is paramount. Heterogeneity of infiltrate density was analyzed with an automated immune cell quantification across complete sections with necrotic areas and artifacts being removed beforehand. In microsatellite stable colorectal cancer, primary tumors showed significant heterogeneity which was also seen in metastatic lesions [46, 48]. Interestingly, systematic analyses in a small number of recurrent metastatic lesions showed that even under prolonged periods of intercurrent chemotherapy, infiltrate densities can remain stable. On the other hand, a recurring lesion after radiofrequency ablation of another liver metastasis showed enhanced immune cell infiltration, suggesting the possibility to convert poorly infiltrated lesions into highly infiltrated lesions [49].
37.5 Primary Tumor and Metastases: Two Different Worlds
Another important aspect is whether the primary tumor situation is reflected in the metastatic situation as well. Little data is available from systematic analyses of colorectal cancer metastases in different metastatic sites (lung versus liver). It is clear however from the available data, that there are organ-specific differences on multiple levels [50]. Data from colorectal and renal cell cancer shows a major prognostic value of the immune pattern of CD8 positive to DC-LAMP positive (for antigen-presenting cells) cell densities in colorectal carcinoma and RCC that was reproducible from primary to metastatic lesions in the lung. The clinical impact was however quite opposite between colorectal and renal cell cancer, which shows the complexities in this area [51]. But is the immune cell infiltration pattern preserved between primary and metastatic sites? In a set of primary colorectal cancer and corresponding liver metastases samples, a ~70% concordance was found for the presence of a “good” or “poor” prognostic immunological signature in the primary and in the metastatic lesion [52]. The factors that drive the heterogeneity in immunologic infiltration in primary and in metastatic lesions have not been elucidated so far and research in this direction is ongoing. Another pressing question is how a tumor lesion of a patient with no or little immune infiltration can be turned into a highly infiltrated lesion.
37.6 Immune Cells and Therapy: Immunogenic Cell Death and Beyond
But even further: what is the mode of action of these T cells in the primary tumor and in the liver metastasis that leads to an improved clinical course of patients? Especially for the effects in the context of chemotherapy, multiple explanations are possible. Activating effects of chemotherapeutic drugs on immune cells with improved antigen presentation and activation of effector T cells is one of the explanations. This could also result in an additional effect of chemotherapy with reduction of the immunosuppressive microenvironment in the tumor. On the other hand, another elegant concept is showing “immunological cell death” induced by selected chemotherapeutic agents. Chemotherapeutic agents such as oxaliplatin are able to stimulate the immune system by inducing a more antigenic and activating tumor cell death [53, 54]. This activation involves the creation of particularly favorable epitopes for antigen presentation on the relevant cells (e.g., dendritic cells), which in turn renders chemotherapy particularly effective [55]. Chemotherapy has also other effects, which are now investigated on a more translational scale. Several groups have shown independently the relationship between the intestinal microbiome and the success of chemotherapy and immunotherapy [56–61]. The mouse model data shows that chemotherapy induces a translocation of bacteria from the intestine across the mucosa and into the lymph nodes where these lead to an enhanced immune stimulation and recognition of the tumor cells. The composition of the bacteria has a decisive role in this process: certain species are relevant as they either enforce an improved immune response or lead to an inhibition. So the composition or the presence of particularly activating bacterial species in the intestine plays an important role for therapy response [62]. Currently, translation into an interventional trial is ongoing. “Oncomicrobiomics” is the concept of [63–66] microbiome modulation to enhance and optimize therapy response.
37.7 Myeloid Immune Cells in the Microenvironment: Plasticity and Immunosuppression
From a quantitative perspective, myeloid cells are the largest immune cell subgroup present in colorectal cancer, especially in metastatic lesions. Macrophages are a versatile and highly complex subgroup of innate immune cells, and their multiple functions are reflected in their enormous plasticity. Besides tissue-resident macrophages, recruited monocytes differentiate into macrophages within the tissue. It is unclear today, whether tumor cell-associated macrophages are arising from tissue-resident or recruited macrophages. Functionally, macrophages with their phagocytic, immunosuppressive (e.g., arginase and iNOS production), and antitumoral (reactive oxygen species formation) capabilities were initially grouped into tumor-promoting M2 and antitumoral M1 categories [65–67]. This however was soon revised, and the complexity of phenotypes of human myeloid cells is not only different from animal models but also highly diversified [66, 68–70]. Nevertheless, interventional strategies with the aim of ablating or modulating macrophages have reached translation into clinical trials [71, 72]. Another important group are the myeloid-derived suppressor cells (MDSCs). This population is typically defined as an immature myeloid cell from the bone marrow with suppressive effects on the adaptive immune response and modulation of other myeloid cells like macrophages [70]. Another important functional effect of MDSCs is the depletion of l-arginine from the microenvironment. Arginase that is produced by MDSCs is degrading l-arginine to l-ornithine and urea and subsequently leads to T cell inactivation. In this continuum of functions, precise separations between different cellular myeloid subclasses and the transient functional states that can be found are not yet possible.
37.8 Checkpoint Inhibition and Other Signaling Cascades
Closely related to these cells are the presence and absence of specific signaling molecules. Molecules of the “programmed death” family with the receptors PD-1 and PD-2 and the ligand PD-L1 (B7-H1) are a part of an increasing family of signaling molecules that shape the functional behavior of all classes of immune cells in the microenvironment. PD-1 is a protein (55 kD) with an extracellular domain showing 23% identity to cytotoxic T lymphocyte antigen-4 (CTLA-4), another prominent inhibitory receptor on activated T cells. PD-1 plays a complex role in the selection of T cells in the thymus and in the peripheral blood. Expression levels of PD-1 on naive T and B cells are low, and only with activation an increased expression level on the surface appears. Data from mouse models suggests a protective role of PD-1 against autoreactivity. Interaction of PD-1 with its ligands leads to reduced levels of cytokines of T-helper cells and subsequent suppression of T cell proliferation [73]. This is apparently an important mechanism in viral infections, where a focusing role of PD-1 was also noted. Looking into cancer, the complexity of the presence of these signaling molecules in the local microenvironment is far from being understood. Following the initial descriptions of the functions of the PD-1/PD-L1 axis as a response to interferon [74], still up to date the complexity and regulation of the expression of PD-L1 on a multitude of immune cells are not fully understood [72, 75, 76]. In colorectal cancer liver metastases, practically all lymphocytes are PD-1 positive; tumor cells are negative for PD-L1 with a specific myeloid cell at the invasive margin being positive for PD-L1 [72]. Clinically, inhibition of the PD-1/PD-L1 axis in colorectal cancer has not shown promising results [77]. But there are also other modulatory molecules present (sometimes specifically at the invasive margin): V-domain immunoglobulin (Ig)-containing suppressor of T cell activation (VISTA) [78], cytotoxic T lymphocyte antigen-4 (CTLA-4) [79], tumor necrosis factor receptor superfamily member 4 (TNFRSF4, also known as CD134 and OX40) [80], T cell immunoglobulin and mucin domain containing 3 (TIM3) [81], lymphocyte activation gene 3 protein (LAG3), and others. The modulation of immune responses for the adaptive immune system has been elucidated [82] for the majority of those receptor-ligand pairs. But there are still plenty of uncertainties, whether there are other receptors/ligands involved and how the coordination in the local microenvironment shapes the actual immunological outcome, especially in human patients. The most prominent question currently is how combination therapies can improve clinical responses and what combinations are most likely successful in a clinical setting.
37.9 Cytokines and Chemokines in the Microenvironment
Beyond the quantitative presence of immune cells and modulatory signals, the role of cytokines and chemokines within the microenvironment holds another potential for therapeutic intervention. The composition of the immune cells or cells at all in the local tumor environment is to a large share affected by these specific molecules. In addition to the classical molecules like interferon-gamma and interleukin-2, there is a wide range of proteins altering the behavior of the immune system, and these have subsequent dramatic effects on tumor cells. In this situation there are many complex relationships still completely unclear, and the pioneering work of the research group of Wolf H. Fridman has elucidated clearly: the immunological context is the decisive factor [83]. A particularly interesting example in this context is the role of IL-10, in particular the work that lead to the discovery of interleukin-10, but also the large number of subsequent publications on the importance of IL-10 for tumor diseases. For a long time, IL-10 was almost uniformly attributed with immunosuppressive and thus tumor-promoting effects. Thus, IL-10 became one of the main target molecules for inhibitory strategies to generate an antitumor activity by the immune system. Several publications recently showed very clear: IL-10 has not only immunosuppressive, but also immune stimulating and focusing functions. A complete absence of IL-10 leads to enhanced metastatic spreading and other pro-tumorigenic effects [84]. This shows not only a high context dependency but also that there is a function of inflammatory cytokines that is also dose-dependent. This dose-dependent functionality in context of other cytokine is difficult to study but shows the urgent need for more integrated analyses. These analyses have to incorporate spatial localization and gradients of chemokines and cytokines together with tumor heterogeneity in terms of tumor cell heterogeneity and immunological heterogeneity as these complex functional relationships allow to dissect the differential effects of the underlying patterns [85, 86]. From a functional perspective, cytokines and chemokines can be grouped into patterns that reflect specific immune activation. This includes the TH1, the TH2, and the TH17 group of cytokines. While the TH1 cytokines indicate an activation of effector T cells favor (with elevated levels of IL-2, etc.), a TH2 environment leads, e.g., to the induction of antibody-producing B cells. The TH17 milieu is typically attributed to autoimmunity and gamma-delta T cell activation. In these different contexts, different groups of cells produce clusters of inflammatory cytokines with differential effects on subsets of immune cells. Cytokines, such as macrophage migration inhibitory factor (MIF), can have wide-ranging pleiotropic effects on multiple different systems (i.e., angiogenesis, cell cycle, etc.) and escape simple classification in one of the above systems.
37.10 Clinical Strategies for Immunotherapy of Colorectal Cancer
The understanding of the immunological mechanisms and the interaction between immune cells, tumor cells, and other cells within the microenvironment has dramatically improved, and the complexity of this interaction has been investigated from many sides [87, 88]. The clinical advances in immunotherapy are doubtless revolutionizing oncology. But has this scientific momentum also affected the clinical development of immunotherapies for colorectal cancer? There is no easy answer, as there are clear benefits for the subgroup of microsatellite instable colorectal cancers [89]. But otherwise, in light of the attempts to utilize immunological interventions, one has to concede that colorectal cancer is a challenging entity for immunotherapy [77]. However, there are promising reports that show clinical effects for advanced stage metastatic colorectal cancer patients, regardless of microsatellite stability or BRAF mutation status.
37.11 Vaccination and Dendritic Cell Therapy: Retargeting the Immune System
From a historical perspective, vaccination has been extensively tried for various cancer entities (see Fig. 37.3). In the year 2006 the summary for vaccination strategies in colorectal cancer looked quite sobering. The review by Nagorsen and Thiel [90] comes to the conclusion that the analysis of 527 patients across a broad variety of specific active immunization strategies showed an overall response rate of 0.9%. Humoral immune responses and cellular responses were seen in approximately half of the patients. Other concepts have been tried, using chemoimmunotherapy in combination with vaccination [91]. This approach resulted in clinical effects, but it was not clarified which effect is contributed by chemoimmunotherapy and which by the vaccination approach.
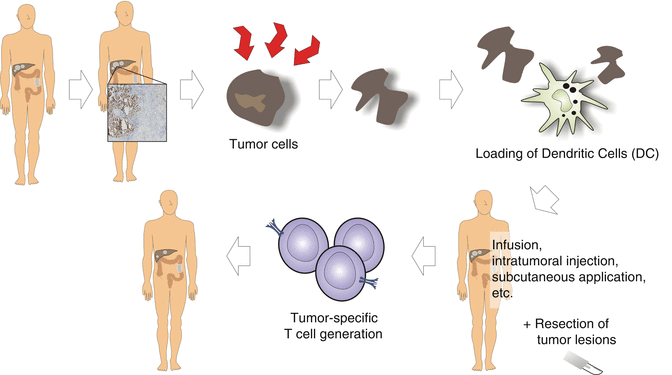

Fig. 37.3
Vaccination strategies for colorectal cancer
Modulation of the immune system with quickly matured dendritic cell vaccines for carcinoembryonic antigen showed no clinical effects [92]. Adjuvant immunization was tried utilizing Newcastle disease modified tumor cells following the resection of the liver metastases in 50 patients [93], but again no clear clinical benefit was identified. An anti-idiotype monoclonal antibody vaccine was also applied to 50 patients following curative resection in the Cancer and Leukemia Group B Study 89,903 [94]. At 2 years, the evaluation showed no benefit for the vaccinated group. A more promising result was seen in a randomized phase II trial, using immunization with dendritic cells modified with poxvectors encoding CEA and MUC1 compared with the same regimen plus GM-CSF in resected metastatic colorectal cancer. Both the dendritic cell and poxvector vaccines showed longer survival for vaccinated patients. A subsequent randomized trial was proposed but no data is available. Interest in vaccination approaches for colorectal cancer has not subsided, and more variations in adjuvants are tried to increase immune cell activation (Garbitsch et al. JCO 32:5 s 2014, Abstract 3093). Given the richness and diversity of approaches in colorectal cancer patients, in summary only for the adjuvant situation, some tangible effects could be observed, no large trials were performed, and for advanced disease the results were disappointing. The Stimuvax trial in colorectal cancer [95] is still ongoing with the trials for lung cancer showing no clinical effects. In 2018 the results for colorectal cancer are expected. In contrast to these “off-the-shelf” vaccines, individualized vaccines seem to be a much more promising new development, and clinical trials are expected to start soon [96].
37.12 Therapeutic Use of Cytokines
Immunomodulation with cytokines has shown interesting results in colorectal cancer. The combination of chemoimmunotherapy with gemcitabine plus FOLFOX-4 followed by subcutaneous GM-CSF and IL-2 (GOLFIG) showed activity in clinical trials. Unfortunately, problems in recruitment for the last trial (for the control arm) led to inconclusive data. Overall, data from this trial series suggests activity of this regimen with objective responses [97, 98]. This combinatorial approach was evaluated with concomitant vaccination [99], utilizing a poly-epitope-peptide vaccine to thymidylate synthase (TSPP). Adverse events consisted of swelling/erythema at injection sites (17 cases), grade I–II hematological alterations (16 cases), and gastrointestinal events (12). Of note, this regimen induced fever, rhinitis, conjunctivitis, polyarthralgia, and a rise in autoantibodies [ANA, ENA, c-ANCA, p-ANCA]. Further clinical evaluations are planned.
37.13 Adoptive T Cell Therapy and Genetically Modified T Cell Therapy
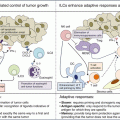
Adoptive T cell therapy is another field of interest for colorectal cancer. In malignant melanoma, spectacular results could be achieved. The principles of the procedure are shown in Fig. 37.4. For colorectal cancer however, adoptive T cell therapy was largely unsuccessful. An adjuvant trial with tumor-infiltrating lymphocytes plus IL-2 after radical resection of liver metastases showed disappointing results in the 5-year follow-up analysis [100]. This approach has been largely abandoned.
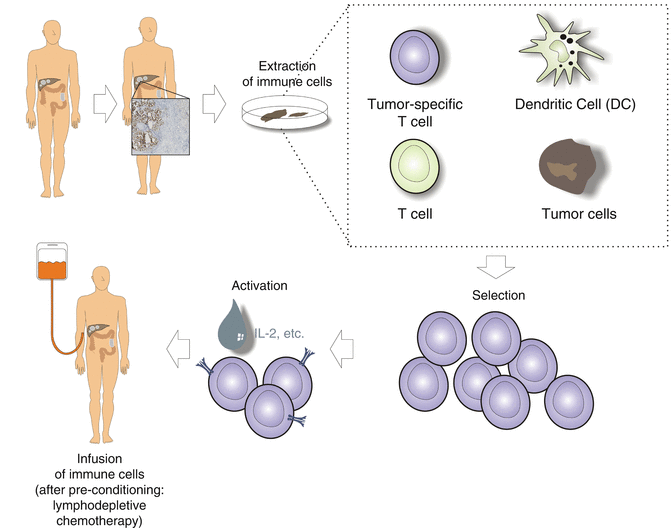

Fig. 37.4
Schematic illustration of adoptive immunotherapy
Another concept is modification of T cell receptors for immunotherapy. The exceptional successes in hematologic malignancies have not been replicated in solid tumors and especially not in colorectal cancer. The genetic modification of T cells to implant a T cell receptor specificity is an elegant method, but apparently these modified T cells succumb to other evasion mechanisms in the microenvironment. Another problem for colorectal cancer seems to be the specificity of the available targets. CEA is also expressed on a variety of other cells, which produces a plethora of unwanted and severe side effects. Severe transient colitis is one of the dominating side effects [101]. The objective regression of metastatic colorectal cancer was followed by unacceptable toxicities. Finding suitable targets and better mechanisms for controlling modified chimeric antigen receptor (CAR) T cells will here allow new opportunities.
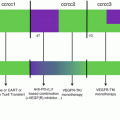
37.14 Checkpoint Inhibition in Colorectal Cancer
Immunomodulation with checkpoint inhibition has been very successful for microsatellite instable (MSI) colorectal cancer, but for the vast majority of patients with microsatellite stable disease (MSS), checkpoint inhibition has been unsuccessful. While data for MSI tumors in general show a favorable situation with good responses across different cancer entities, for MSS colorectal cancers, checkpoint inhibition with anti-PD-1, anti-PD-L1 [102], or anti-CTLA-4 has not shown efficacy. The checkmate 142 trial combines anti-PD-1 (nivolumab) with anti-CTLA4 (ipilimumab) inhibition in colorectal cancer patients. Sure enough, patients with MSI tumors showed responses whereas the patients with MSS tumors did not benefit.
Combination of anti-PD-L1 with small molecule inhibition (MEK inhibitor) from an early phase I trial showed promising results (see Fig. 37.5). From the reported 23 CRC patients (22 KRAS mutant, 1 WT), the most common treatment-related side effects included diarrhea (69.6%), fatigue (52.2%), acneiform dermatitis (43.5%), rash (34.8%), maculopapular rash (26.1%), pruritus (26.1%), and nausea (26.1%). The incidence of treatment-related grade III and IV adverse events was 34.8%, more than two patients developed severe diarrhea (8.7%). However, no grade V adverse events were reported. The ORR was 17% with four partial remissions and five stable diseases. Interestingly, three responses were reported to be still ongoing (range, 4.0–7.7 months at time of data cutoff). As the trial was aiming at microsatellite stable patients, three responders were mismatch repair proficient, and in one patient the status was unknown. In contrast to expectations, response was not associated with baseline PD-L1 expression. To obtain a better image of the effects of therapy on the microenvironment, serial biopsies were taken and showed enhanced PD-L1 upregulation, CD8 T cell infiltration, and enhanced MHC class I expression. This finding is in line with the previous report of the unexpected observation that potent suppression of T cell receptor (TCR) function by MEK inhibition can be largely overcome in the presence of blockade of the inhibitory PD-L1/PD-1 pathway in T cells in vivo. Enhanced antitumor activity was observed combining MEK inhibition with PD-L1 blockade in in vitro experiments, which was likely potentiated by upregulation of tumor MHC class I expression through inhibition of MEK [103]. This therapeutical concept is currently evaluated in a phase III trial (NCT02788279).
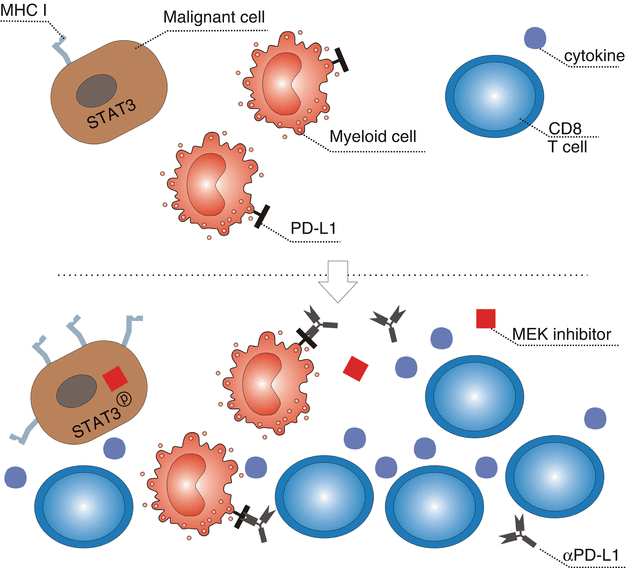

Fig. 37.5
Effects of combined MEK inhibition and anti-PD-L1 therapy in the microenvironment
37.15 Modulation of the Innate Immune System
In contrast to immunotherapies that aim to activate the adaptive immune system, a new form of immunotherapy aims at immunomodulation of innate immune cells: modulation of macrophage (MOMA) therapy. This modulation is contrasting to depleting approaches like bisphosphonates or depleting antibodies (anti-CSF-1R) [71] and utilizes the innate arm of the immune system against the tumor [104].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree