Cervical cancer stem cell marker
Function of marker
Sample
% of marker positivity
Characteristics of CSC isolated
References
CK17 p63
CK17 – differentiation marker p63 – TF activated at embryonic development stages
Normal CIN I, II, II
Basal cells/CIN lesions – p63: 76–100 %, CK17: 26–50 %
Both CK17 and p63 overexpressed in basal cells, a site for resident tissue stem cells
Martens et al. (2004)
Nanog Nucleostemin Musashi1
Nanog – TF maintain self-renewal Nucleostemin- regulate cell cycle and effect cell differentiation Musashi1 – posttranscriptional gene regulation
Cervical cancer tissue
Normal tissue <65 % and Cancerous tissue >90 %
Regulate cell differentiation, proliferation, asymmetric division, and maintain cancer cell pluripotency
Ye et al. (2008)
CD44+, CK17+ Sphere formation, Xenograft assay
CD44 – cell adhesion and migration Spheres – contact independent growth Xenograft – tumorigenic potential
Cervical cancer tissue
Not defined
Promote self-renewal, chemoresistance, in vivo tumorigenicity, ABCG2, Stat3, Oct4, Piwil, c-Myc overexpressed
Feng et al. (2009)
Sox-2 Xenograft assay
Sox-2 – TF maintains self-renewal and pluripotency
Normal, CIN I-II-II, cervical squamous cell carcinoma, cell line
Normal cervix, 25 %, CIN III, 83 %, carcinoma, 77 %
Increased proliferation, clonogenicity, and tumorigenicity in vitro and in vivo
Ji and Zheng (2010)
Sphere ALDH1 SP
Spheres – contact independent growth ALDH1 – oxidation of aldehydes to carboxylic acid. Cytoprotection of stem cells SP – functional ABCG2
CaSki, SiHa, A431 cell lines
A431 sphere-ALDH- 64.9 % A431Sphere-SP-1.2 %
High sphere forming efficiency, adherent holoclone formation, CD44, and ALDH do not identify specific subpopulation with TIC properties. Nanog, Nestin, Oct4 overexpressed
Bortolomai et al. (2010)
ALDH1
ALDH1 – oxidation of aldehydes to carboxylic acid. Cytoprotection of stem cells
Cervical cancer tissue
Invasive carcinoma, 25 %; adenocarcinoma, 20 %
ALDH1 expression linked to the cancer stem cells
Yao et al. (2011)
Twist Spheres, ALDH1, CD44
Twist – TF induce epithelial mesenchymal transition ALDH – Cytoprotection of stem cells
HeLa cells
Twist overexpressed
Twist expression lead to elevation of CD44 and ALDH
Li and Zhou (2011)
Sphere Xenograft assay
Spheres – contact independent growth CD44 – cell adhesion and migration CD24 – cell adhesion SP – functional drug transporter ABCG2
HeLa cells
At early passage, 15–20 % Late passage, 6 %
High tumorigenicity and expression of CD44, chemoresistance
Gu et al. (2011)
Sphere, CD49f
CD49f – cell adhesion, cell surface-mediated signaling
HeLa, SiHa, CaSki, and C-4I cell lines
4–12 %
High radioresistance and increased expression of epithelial-mesenchymal transition markers
Lopez et al. (2012)
Side population (SP) Xenograft assay
SP – functional drug transporter ABCG2
HeLa cell line
1.2 %
High tumorigenicity, chemo-/radioresistant, CD133 expression, no difference in CD44 expression
Wang et al. (2013a)
SP Xenograft assay
SP – functional ABCG2
HeLa
Not available
Sensitive to chemotherapy in the presence of ABCG2 inhibitor in vitro and in vivo
Shishido et al. (2013)
SP
SP – functional ABCG2
CALO, SiHa, and C33a cell lines
3 % (in CALO, SiHa only)
Higher clonogenic potential to form holoclones, greater colony-forming efficiency under anchorage-independent growth conditions alpha6-integrin bri/CD71dim pattern in SP
Villanueva-Toledo et al. (2014)
Oct-4 Xenograft assay
Oct-4 – self-renewal of undifferentiated embryonic stem cells
Cervical cancer tissue and primary cultures
Oct-4 overexpressed
Differentiation and metastasis
Yang et al. (2014)
Sox-2 Sphere Xenograft
Sox-2 – TF maintains self-renewal and pluripotency
SiHa and C33a cell lines
Sox-2 overexpressed
Greater capacities for self-renewal, differentiation, and tumor formation
Liu et al. (2014)
SP Xenograft assay
SP – functional drug transporter ABCG2
Xenograft – Tumorigenic potential
HeLa cells
1.07 %
High proliferation ability, chemo-/radioresistant, High expression of Oct3/4, CD133, BCRP
Qi et al. (2014)
A major bottleneck in understanding the phenotypic and molecular signatures of cervical CSC is their low frequency and the ability to quickly shift to a more differentiated phenotype. Attempts made using repeated culturing of cervicosphere cells in low adhesion environment have been only marginally effective in isolation and enrichment of CSCs (Lopez et al. 2012). Among different approaches tested for identification and isolation of cervical CSC, side population assay based on Hoechst or dye cycle violet dichromatic dye exclusion principle that target functionally active ABCG2 (and the cells are known as side population or SP cells) is emerging as most popular experimental procedure to isolate cervical CSC. Conventionally, the SP cells isolated showed high degree of tumorigenicity in vitro and in vivo (Patrawala et al. 2005). Interestingly, a recent study has demonstrated that non-SP cells are also tumorigenic (Qi et al. 2014). These observations suggest that SP-based approach to gate CSCs is only partially effective. Therefore, further research is needed to develop more efficient and robust methods for CSC isolation. In such a scenario, a combination of side population assay along with other stem cell-related markers that have direct impact on stem cell signaling could be more effective and should be standardized for enrichment of cervical CSC.
24.5 Cervical Stem Cells as Target of HPV Infection
Apart from identification of cervical cancer stem cells, there is no information on the impact of HPV infection or its oncoproteins on putative CSCs proportions or on the stem cell signaling and downstream functions. It is hypothesized that HPV infects the basal cells residing within transformation zone where columnar cells of endocervix meet the squamous cells of ectocervix (Martens et al. 2004; Cid Arregui et al. 2012). This region which is known as squamocolumnar junction (SCJ) is suggested to generate stem cells that maintain columnar and stratified epithelial cells of the cervix (Martens et al. 2009). SCJ cells are known to express high levels of cytokeratin CK17. Infection of these slow-cycling epithelial cells is believed to be necessary for the infection to persist over a long period, which can eventually integrate to induce tumorigenic transformation and cancer. However, till date there is no experimental evidence to validate the hypothesis. A recent study demonstrates that there exists a discrete population of cells at the SCJ of the cervix with a unique gene expression profile that could be responsible for most HR-HPV-associated cervical carcinomas (Herfs et al. 2012). The selected junctional biomarkers proposed, i.e., keratin Krt7, anterior gradient AGR2, cluster differentiation marker CD63, matrix metalloproteinase MMP7, and guanine deaminase (GDA), were expressed by SCJ cells. These markers were found to be expressed by a high percentage of high-grade CIN and cervical cancers associated with carcinogenic HPVs but rarely in ectocervical/transformation zone CINs. Interestingly, these markers displayed on these SCJ are also displayed by some most common cervical cancer cell lines SiHa, HeLa, and CaSki, suggesting that these cell lines do serve as suitable in vitro models for the SCJ cells to be used in experimental procedures. These markers, however, do not confirm stemness and were not associated particularly with CSC as majority of cervical precancer or cancer cells expressed them. Stemness and differentiation being the two extremes of a gradient, it is likely that studying the quantitative gradient of stemness-related markers would be helpful in finding CSC in situ in a tumor tissue. Nevertheless, these studies provide essential leads demonstrating a potential tropicity of HPV towards a set of cells with probable stem cell characteristics that reside in SCJ region of transformation zone. If SCJ cells or the resident stem cells are the true targets of HPV, it will be quite interesting to determine how HPV modulates the stem cell signaling in these cells. Recent advancement in technology has made it possible to study the life cycle of HPV in vitro by organotypic raft culture that has been an important tool to study HPV replication and its interactions with the host cell. An immortalized human foreskin keratinocyte (HFK) cell line, BC-1-Ep/SL, when stably transfected with HPV16 can sustain productive stages of HPV16 life cycle in organotypic raft culture system (Flores et al. 1999). Using these in vitro models, the progression of low-grade intraepithelial lesions (LSIL) to high-grade intraepithelial lesions (HSIL) can be studied (Isaacson Wechsler et al. 2012). Such organotypic raft culture system can open up an avenue to understand the target cells of HPV infection and the mechanism of generation of cervical CSC. These experiments can also reveal the contribution of HPV oncoproteins in regulation of stem cell signaling pathway and other stemness-related transcription factors, reported to be expressed in cervical cancer.
24.6 Stem Cell Signaling in Cervical Cancer
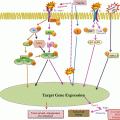
Stem cell property is highly dynamic in nature and conserved even in tumor tissues. A homeostasis that determines the proportion of CSC or non-CSC is believed to be maintained in tumors despite tumorigenic transformation (Reya et al. 2001). Both normal stem cells and CSC resemble in their stemness properties and are proposed to be controlled by the same/similar self-renewal pathways that control stemness in normal stem cells (Fig. 24.1). These stemness-related signaling pathways are broadly classified as Notch, Hh, and Wnt signaling pathways (Pardal et al. 2003). These pathways primarily govern stem cell fate determination, self-renewal, and differentiation. The signaling events act in tandem along with a set of transcription factors like Sox2, Oct4, Nanog, and STAT3 that have been implicated with stemness, self-renewal, pluripotency, and nuclear reprogramming (Tam and Lim 2008). The balance between Wnt, Notch, and Hh signaling networks is important for the maintenance of homeostasis among stem and progenitor cells. Aberrant activation of these pathways has been frequently implicated in progression of many tumor types (Dreesen and Brivanlou 2007; Takebe et al. 2011) including cervical cancer (Table 24.2). Reports indicate activation of Notch, Hh, and Wnt pathways to be necessary for the maintenance of normal cervix and subsequent tumor progression (Zagouras et al. 1995; Daniel et al. 1997; Gray et al. 1999; Shinohara et al. 2001; Okino et al. 2003; Xuan et al. 2006). In independent studies, expression of these pathways have been highly correlated with the progression of cervical cancer with their tendency to increase with the tumor progression from normal to CIN and cervical cancer, indicating gross disturbance in these signaling pathways during cancer progression. However, the orchestration of these pathways, their hierarchy and cross-talk between each other in regulation of cervical CSCs and cellular homeostasis, is not well defined. Cell fate of proliferating stratum spinosum and reserve cells of squamous epithelium that differentiate into squamous and columnar cells respectively in normal cervix have been hypothesized to be determined by Notch signaling via differential expression of Notch1/2 (Zagouras et al. 1995). Overexpression of Notch in these cells might be responsible for generating CSC in cancer as the overexpression of Notch1, Notch2, and Notch intracellular domain (NICD), along with their ligands Jagged1 and Jagged2, have been implicated in progression of cervical cancer (Daniel et al. 1997; Gray et al. 1999; Weijzen et al. 2003). Likewise, Hes1 and Hes5, the downstream target of Notch signaling, are suggested to inhibit differentiation and promote cell proliferation in cervical cancer (Liu et al. 2007, 2010) but their role/status in cervical CSC is yet to be ascertained. Apart from Notch pathway, the overexpression of nuclear β-catenin, a central mediator of Wnt pathway, has been correlated with the progression of cervical cancer (Shinohara et al. 2001). Later, it was shown that overexpression of DVL-1, an upstream regulator that prevents sequestration of β-catenin, resulted in constitutively active Wnt signaling and promoted tumorigenesis (Okino et al. 2003). High nuclear expression of GLI, the downstream mediator of Hh, and other components of this pathway are overexpressed in cervical cancer and have been implicated in tumor recurrence (Xuan et al. 2006; Chaudary et al. 2012). These studies represent individual contribution of signaling pathways in cervical cancer. Further studies are needed to understand the extent of contribution of each signaling pathway in regulation of CSC in an in vivo situation.
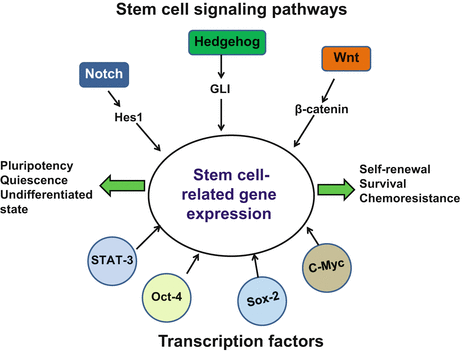

Fig. 24.1
Overview of major stem cell signaling pathways and transcription factors that control properties and functions of normal stem cells and cancer stem cells
Table 24.2
Status of stem cell-related signaling pathways in cervical cancer and their association with HPV
Signaling pathways | Mediators | Sample type | Signaling status in cervical tissues | HPV association | References |
|---|---|---|---|---|---|
Notch | Notch1 (15A) and Notch2 (6D) and TLE(4A), homologue of Groucho | Normal and cervical cancer tissue | Normal: squamous cells express nuclear Notch2. Reserve cells express Jagged1 and Delta1 | Not defined | |
Cancer: metaplastic cells express nuclear Notch1/2, TLE and Jagged1. Reserve cells express nuclear Notch1/2 and TLE | |||||
Human Jagged1 (HJ1), Human Jagged2 (HJ2), and Human Delta1 | |||||
Conclusion: expression of Notch, TLE proteins coincide during normal and metaplastic cell-fate decision. Inappropriate activation or inactivation of Notch pathway derail cells differentiation potential | |||||
Notch | Cervical precancer and cancer tissues | Normal: not studied | HPVE6/E7 transcript increase in cancer as compared to precancer lesions with increased expression of NICD | Daniel et al. (1997) | |
Precancer: early CIN lesions (77 %) – not detectable | |||||
Cancer: late CINIII lesions (75 %) – membrane to cytoplasmic | |||||
CIS (100 %) – nuclear | |||||
Conclusion: increases in expression level of E6/E7 transcripts, coupled with changes in the cellular localization of the Notch protein, define the transition from CIN III lesions to tumors | |||||
NICD | Human and mouse primary cell lines | Conclusion: Notch1 protein localization changes from cytoplasmic to nuclear with the transition from CINIII to microinvasive carcinoma | HPVE6/E7 regulate Notch1 | Weijzen et al. (2003) | |
Presenilin | |||||
Hes1/Hes5 | Normal, precancer cancer tissues and cervical SiHa cell line | Normal: nil to low expression | Not defined | ||
Precancer: early CIN lesion, low nuclear Hes1 and Hes5; late CIN lesions, high nuclear Hes1 and Hes5 | |||||
Cancer: nuclear Hes1 and Hes5 expression | |||||
Conclusion: Hes1 and Hes5 predict poor prognosis in early cancer cases. Hes1/Hes5 gene inhibit the cell differentiation and promote the cell proliferation in cervical carcinoma cells | |||||
Wnt/beta catenin | Beta catenin | Normal, cervical precancer, cancer tissue | Normal: 100 % membranous in basal and parabasal cell | Not defined | Shinohara et al. (2001) |
Precancer: 28 % cytoplasmic in basal and parabasal cell | |||||
Cancer: 46 % cytoplasmic as well as nuclear throughout the epithelium | |||||
Dishevelled (DVL-1) | Cervical cancer tissue and cell lines | Overexpression of DVL-1 in 66 % of primary cervical cancer | Not defined | Okino et al. (2003) | |
Upregulation of the DVL-1 gene is involved in tumorigenesis of cervical cancer | |||||
Hedgehog | Shh | Normal and cervical cancer tissues pre-/post-chemo-/radiotherapy | Normal: nil to low expression of Hh-signaling molecules | Not defined | |
Ihh | |||||
Patched | Precancer and cancer: strong cytoplasmic expression of all Hh-signaling molecules except Gli-1/2 expressing both in cytoplasm and nuclear | ||||
Smo | |||||
Gli-1/2/3 | |||||
Post-chemoradiotherapy cancer cases: low expression of PTCH1/PTCH2 and SMO while high expression Shh/Ihh/Gli in advanced stage | |||||
Conclusion: exaggerated and inappropriate activation of Hh signaling pathway in HPV-infected cervical cancer may exert a synergistic during cervical carcinogenesis and could be responsible for local tumor recurrence | |||||
Shh | Cervical cancer cell lines | Shh ligand induces proliferation and promotes migration of the cervical cancer cells indicating existence of autocrine signaling loop | Hh-activating mutation are selected in HPV immortalized cells | Samarzija and Beard (2012) |
Similar to majority of the signaling pathways, there is an evidence of the activation of stemness-related transcription factors c-myc, Sox2, Stat3, and Oct4 during cervical cancer progression (Abba et al. 2004; Ji and Zheng 2010; Liu et al. 2010; Shukla et al. 2010; Wang et al. 2013b) (Table 24.3). c-myc, well known for its capabilities of cellular reprogramming, was found amplified in HPV positive precancer and cancer lesions (Abba et al. 2004). Sox2 was found localized in nucleus of highly tumorigenic primary cervical cancer cells (Ji and Zheng 2010). Active STAT3 required for early embryonic stem cell potency and self-renewal (Raz et al. 1999) was found increased with increasing severity of the cervical lesions (Shukla et al. 2010, 2013). Similarly, Oct4, a key regulator that maintains the pluripotency and self-renewal properties of embryonic stem cells, was overexpressed in cervical lesions and was associated with lymphatic metastasis (Wang et al. 2013b). Experimental or intrinsic overexpression of transcription factors such as Sox2 or Oct4 in cervical cells was found to increase cells with CSC phenotype (Liu et al. 2014; Yang et al. 2014). Interestingly, stemlike cells isolated on CD44/CK17 marker from primary cervical carcinoma tissues also showed elevated levels of c-myc, Sox2, STAT3, and Oct4 transcript in the spheroid cells, thus suggesting their role in cancer stem cell signaling (Feng et al. 2009). Collectively, these studies clearly demonstrate the presence of CSC as well as an active CSC signaling in cervical cancer tissues.
Table 24.3
Status of stem cell-related transcription factors in cervical cancer and their association with HPV
Transcription factor | Mediators | Sample type | Status in cervical tissues | Relation with HPV | Speculation w.r.t cervical CSC | References |
|---|---|---|---|---|---|---|
c-Myc | c-Myc copy no. | Normal and cervical cancer tissues | Overexpressed in 39–69 % of HR-HPV16 infected cases with increasing severity of lesions from LSIL to cancer | c-Myc amplification is associated with HR-HPV16 infection | Not defined | Abba et al. (2004) |
Sox2 | Nuclear Sox2 | Cervical cancer biopsies and primary culture | Normal: no expression | Not defined | Overexpressed in tumorosphere with high tumorigenicity | Ji and Zheng (2010) |
Cancer: nuclear expression | ||||||
Stat3 | pSTAT3 (nuclear) | Normal and cervical cancer tissue, cervical cancer cell lines | Precancer: active pStat3 in basal layer cells | High-risk HPV16 infection in cervical lesions is significantly correlated with active STAT3 expression. High levels of E6 and E7 correlate with high STAT3 activity | Not defined | Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|