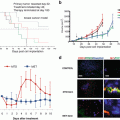
Fig. 10.1
Drug-efficacy testing in the PDOX model. PDOX nude mice were treated with saline, carboplatin, trastuzumab + lapatinib, trastuzumab + lapatinib + entinostat, or entinostat alone. (a) Bar graphs of the primary tumor weight in each group. (b) Bar graphs of the metastatic tumor weight in each group. (n = 5 for each treatment arm). *p<0.05; **p<0.01
The efficacy of entinostat on the metastasis and not the primary cervical PDOX tumor or subcutaneous tumor is an important example of the critical need of orthotopic models of patient tumors since drugs, such as entinostat, can be evaluated are selectively effective against metastasis only and would have been missed by a subcutaneous model.
PDOX model of squamous cell cervical cancer: The patient was a 57-year-old female with primary cervical cancer. Histology demonstrated squamous cell carcinoma (grade 2). The patient received no previous treatment. A radical hysterectomy was performed with bilateral salpingo-oophorectomy and pelvic lymphadenectomy [38]. Nanoparticle albumin-bound (nab)-paclitaxel (NAB-PTX) is paclitaxel linked to albumin nanoparticles, which makes it soluble. The development of nanotechnology as a delivery system for NAB-PTX has improved the pharmacokinetics and pharmacodynamics of paclitaxel, in part by decreasing its hydrophobicity [38, 39].
In a this study, we compared the efficacy of two first-line drugs for cervical cancer, cisplatinum (CDDP), and NAB-PTX [38]. CDDP was highly effective. One tumor treated with CDDP completely regressed. CDDP-treated tumors were significantly smaller (tumor volume ratio, 0.42 ± 0.36) than untreated control mice. In contrast, NAB-PTX did not show efficacy on the cervical cancer PDOX model. CDDP-treated tumor weight was significantly less than control. NAB-PTX-treated tumors were not reduced in weight compared to control. There were no significant differences in mouse body weight between groups. Histological evaluation demonstrated that CDDP-treated tumors were fibrotic with scattered squamous cell nests compared to those of control or NAB-PTX-treated [38]. The PDOX model in this case showed that an inexpensive drug, CDDP, was more effective than a very expensive drug.
Materials and Methods
Establishment of Patient-Derived Cervical Cancer
Orthotopic Tumor Implantation
After the subcutaneous tumors grew in the nude mice, they were harvested and divided into small fragments for orthotopic transplantation which was performed as follows: a small 6–10-mm midline incision was made on the lower abdomen of the nude mouse through the skin and peritoneum. The uterus was exposed through this incision, and a single 3-mm3 tumor fragment was sutured to the cervix of the uterus using 8-0 nylon surgical sutures (Ethilon, Ethicon Inc., NJ, USA). On completion of tumor implantation, the uterus was returned to the abdomen, and the incision was closed in one layer using 6-0 nylon surgical sutures (Ethilon) [37].
Treatment
Six weeks after implantation, the mice in the PDX and PDOX models of the HER-2-positive cervical cancer were randomized and treated in the following groups of n = 5:
- 1.
Saline (vehicle/control, ip, weekly, 5 weeks)
- 2.
Carboplatin (Selleck Chemicals, Houston, TX, USA, 30 mg/kg, ip, weekly, 5 weeks)
- 3.
Trastuzumab (Genentech, Inc., South San Francisco, CA, USA, 20 mg/kg, ip, weekly, 5 weeks) + lapatinib (Selleck Chemicals, 100 mg/kg, orally, daily, 5 weeks)
- 4.
Trastuzumab (20 mg/kg, ip, weekly, 5 weeks) + lapatinib (100 mg/kg, orally, daily, 5 weeks) + entinostat (Selleck Chemicals, 5 mg/kg, orally, daily, 5 weeks)
- 5.
Entinostat (5 mg/kg, orally, daily, 5 weeks)
For the subcutaneous model, tumor size was evaluated every 3 or 4 days by caliper measurements, and the approximate tumor volume was calculated using the formula 4/3π × (d/2)2 × D/2, where d is the minor tumor axis and D is the major tumor axis [37].
In another PDOX model of cervical cancer, treatment was as follows: treatment protocol (G1, control treated with vehicle (i.v. phosphate buffered saline (PBS), once a week, 3 weeks, n = 7); G2, treated with CDDP (i.v., 5 mg/kg, once a week, 3 weeks, n = 7); G3, treated with NAB-paclitaxel (NAB-PTX) (i.v., 10 mg/kg, twice a week, 3 weeks, n = 7)). Treatment started 4 weeks after orthotopic implantation. Mice were sacrificed on day 22; then tumors were resected for further evaluation. Tumor length and width were measured both on day 0 and day 22. Tumor volume was calculated by the following formula: tumor volume (mm3) = length (mm) × width (mm) × width (mm) × 1/2. Tumor volume ratio was defined as the ratio of volume on day 22 to day 0 [38].
For the orthotopic model, the mice underwent laparotomy 1 week before treatment to confirm the presence of the primary tumor, and its size was evaluated as described above [37].
Relative tumor volume and body weight were calculated by comparison to tumor size before treatment. Animals underwent laparotomy after treatment, and the tumors were photographed with a Canon EOS 60D digital camera with EF-S 18-55 IS lens (Canon, Tokyo, Japan) and weighed and harvested for analysis. Body weight of the mice was measured in a balance once a week [37].
Tissue Histology
Tumor samples were removed with surrounding normal tissues at the time of resection. Fresh tissue samples were fixed in 10% formalin and embedded in paraffin before sectioning and staining. Tissue sections (3 μm) were deparaffinized in xylene and rehydrated in an ethanol series. Hematoxylin and eosin (H&E) staining was performed according to standard protocols. For immunohistochemistry, the sections were then treated for 30 min with 0.3% hydrogen peroxide to block endogenous peroxidase activity. The sections were subsequently washed with PBS and incubated in citrate antigen unmasking solution (Mitsubishi Kagaku Iatron, Inc., Tokyo, Japan) in a water bath for 40 min at 98 °C. After incubation with 10% normal goat serum, the sections were incubated with anti-HER-2/ErbB2 antibody (1:100, Cell Signaling Technology, Danvers, MA, USA) at 4 °C overnight. The binding of primary antibodies was detected using anti-mouse secondary antibodies and avidin/biotin/horseradish peroxidase complex (DAKO Cytomation, Kyoto, Japan) for 30 min at room temperature. The labeled antigens were visualized with the DAB kit (DAKO Cytomation). Finally, the sections were counterstained with hematoxylin and examined using an Olympus BH-2 microscope equipped with a INFINITY1 2.0 megapixel CMOS digital camera (Lumenera Corporation, Ottawa, Canada). All images were acquired using INFINITY ANALYZE software (Lumenera Corporation) without post-acquisition processing [37].
Statistical Analysis
PASW Statistics 18.0 (SPSS, Inc.) was used for all statistical analyses. The Student’s t-test was used to compare continuous variables between two groups. Analyses of variance models were used to compare multiple groups. A P-value of 0.05 was considered statistically significant for all comparisons [37].
References
1.
2.
Sabatier R, Roussin C, Riviere JP, Jalaguier A, Jacquemier J, Bertucci F. Breast metastasis of a squamous cell carcinoma of the uterine cervix mimicking inflammatory breast cancer. Case Rep Oncol. 2012;5:464–70.CrossrefPubMedPubMedCentral
3.
4.
5.
Hashimoto K, Yonemori K, Katsumata N, Hirakawa A, Hirata T, Yamamoto H, Shimizu C, Tamura K, Ando M, Fujiwara Y. Use of squamous cell carcinoma antigen as a biomarker of chemotherapy response in patients with metastatic cervical carcinoma. Eur J Obstet Gynecol Reprod Biol. 2011;159:394–8.CrossrefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree