E1
E2
E4
E5
E6
E7
L1
L2
ATPase
Regulator of E6 and E7
Disrupts cytokeratin matrix for release of virions
Potentiation of membrane bound EGF receptors
Bind and inactivate p53
Bind pRB leading to E2F activity
Major capsid (conserved)
Minor capsid (variable)
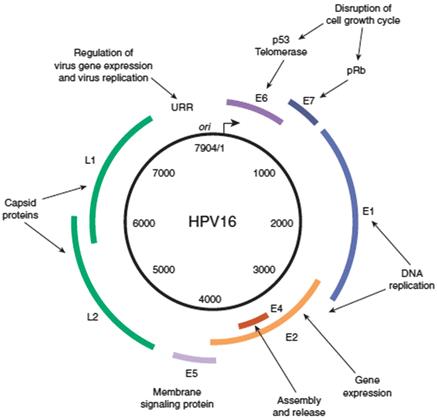
Fig. 2.1.
Human papillomavirus genome [4]. Reprinted from Clinical Gynecologic Oncology, 7th Edition, Di Saia PJ, Creasman WT. Chapter 3 Invasive Cervical Cancer, Monk BJ, Tewari KS, Copyright 2007, with permission from Elsevier. Clinical gynecologic oncology by Di Saia PJ, Creasman WT. Reproduced with permission of Elsevier Mosby in the format reuse in a book/textbook via Copyright Clearance Center.
HPV is detectable in over 95 % of squamous cell carcinomas and 30–40 % of adenocarcinomas.
High-risk strains cause a mutation of cells in the squamocolumnar junction leading to cervical dysplasia and cancer.
Incidence of progression without treatment:
CIN1 (16 %), CIN2 (30 %), CIN3 (70 %).
CIN3 → invasive disease: 0–20 years.
Risk factors:
Lower socioeconomic status.
Multiple sexual partners, early age of first intercourse, promiscuous partners, co-infection with other sexually transmitted diseases.
Tobacco use.
Immunocompromised conditions (HIV or pharmacologic).
The greatest risk for developing cervical cancer is infrequent or no prior screening.
In many South American, African, and Asian countries, cervical cancer is the leading cause of cancer related death in women.
Prevention
Abstinence prevents HPV related cervical carcinomas, but the large majority of women are sexually active and therefore at risk for exposure to HPV infection.
Two US Food and Drug Administration (FDA)-approved vaccines indicated to prevent cervical cancer (Table 2.2).
Table 2.2.
HPV directed vaccines.
Gardasil
Cervarix
HPV types
6,11, 16, 18
16, 18
Dose schedule
0.5 mL IM 0, 2, 6 months
0.5 mL IM 0, 1–2, 6 months
Indications
Cervical cancer, CIN, AIS, Vulvar cancer, VIN, Vaginal cancer, VAIN, Anogenital warts
Cervical cancer, CIN, AIS
Population approved
Males and females aged 9–26 years
Females aged 9–25 years
Advisory Committee on Immunization Practices (ACIP) recommendations
Females aged 11 and 12 years with catch-up vaccination for females aged 13–26 years. Permissive for boys aged 9–26 years
Technology used
Yeast
Insect cell substrate
Adjuvant
Amorphous hydroxyphosphate sulfate (Merck and Co., Inc)
Aluminum hydroxide + 3 = deacetylated monophosphoryl lipid A (MPL, Coixa/GSK)
Quadravalent Vaccine: GARDASIL.
FDA approved in 2006.
In 2007 the Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) II reported results from a randomized, double-blind trial of 12,167 women aged 15–26 years who received Gardasil or placebo. For 3-year follow-up, vaccine efficacy for preventing dysplasia or invasive disease was 98 % in the per-protocol population (44 % for the intention-to-treat population).
FUTURE I was a phase III, randomized, double-blind, placebo-controlled trial involving 5,455 women aged 16–24 years. Vaccine efficacy for preventing anogenital warts as well as dysplasia or invasive disease associated with HPV types 16 or 18 was 100 %.
In a double-blind, randomized trial of 3,817 women aged 24–45 years, GARDASIL efficacy against infection related to HPV-6, -11, -16, and -18 was 90.5 %.
Merck is currently comparing the efficacy of GARDASIL to a nanovalent HPV vaccine.
Bivalent Vaccine: CERVARIX.
Phase II, randomized, double-blind, controlled trial known as Papilloma TRIal against Cancer In young Adults (PATRICIA) published in 2009. In this study, 18,644 women aged 15–25 years received placebo or were vaccinated with CERVARIX.
Vaccine efficacy against HPV-16 and -18 CIN II–IIII was 92.9 %.
Evidence of cross-protection efficacy.
There has not been a direct head-to-head efficacy trial between GARDASIL and CERVARIX.
Diagnosis
First symptom of early cervical cancer: frequently thin, clear or blood-tinged vaginal discharge usually unrecognized by the patient.
Classic symptom: intermittent, painless metrorrhagia or postcoital spotting, although this is not the most common symptom.
With progression, bleeding becomes heavier, more frequent, and ultimately continuous. Usually if this bleeding occurs in a postmenopausal woman, it leads to earlier medical attention.
Late stage disease involves spread into the parametria or the pelvic sidewalls and causes flank or leg pain, which is usually a sign of involvement of the ureters or sciatic nerve. Bladder or rectal invasion frequently leads to hematuria, rectal bleeding, and possibly vesicovaginal or rectovaginal fistula. Lymphedema may be a sign of late stage or recurrent disease due to venous blockage from extensive sidewall disease.
Gross clinical appearance.
Most common: exophytic, large, friable polypoid lesion arising from the ectocervix (Fig. 2.2). These lesions may arise within the endocervcial canal creating a barrel-shaped lesion.
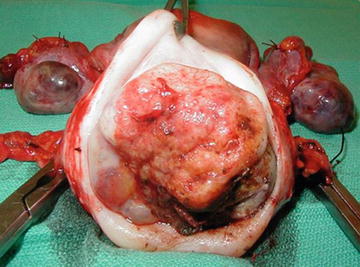
Fig. 2.2.
Gross image of invasive cervical carcinoma (Image provided courtesy of Dr. Krishnansu S. Tewari).
Lesions within the endocervical canal are more commonly adenocarcinomas, which arise in the endocervical mucous-producing gland cells. Because of the origin within the cervix, the lesion may be present for longer time before it is clinically evident.
Firm cervix with little visible ulceration or mass.
An ulcerative tumor that erodes through the cervix.
Screening
Prevention, screening, and early treatment are imperative.
Cervical dysplasia and cancer is slow to progress, able to be diagnosed early with current screening modalities, and almost always cured when diagnosed early.
Late diagnosis most frequently results in incurable disease and death.
Cytology, using the Papanicolaou (Pap) smear, and colposcopy are both valuable screening tools.
Cervical cancer screening guidelines according to American Society for Colposcopy and Cervical Pathology (ASCCP) (Table 2.3).
Table 2.3.
ASCCP cervical cancer screening guidelines.
Population
Screening recommendation
<21 years
No screening
21–29 years
Cytology every 3 years without HPV testing
30–65 years
Cytology and HPV co-testing every 5 years
>65 years
No screening if negative adequate prior screening (as long as no prior history of CIN or cervical cancer)
After hysterectomy
No screening (as long as cervix removed and no prior history of CIN or cervical cancer)
After HPV vaccination
Same as unvaccinated women
Abnormal pap smears may require further workup with colposcopy with possible need for biopsy.
Colposcopy involves use of 5 % acetic acid applied to the cervix and inspection with a colposcope that magnifies the cervix and allows for visualization with color filters.
A satisfactory colposcopy requires that the entire squamocolumnar junction (SCJ) be visualized.
Concerning findings for which biopsy should be obtained:
Acetowhite changes.
Irregular contour.
Atypical vessels.
Coarse mosaicism or punctation.
Large multiquadrant lesions.
An endocervical curettage (ECC) should be done as long as the patient is not pregnant.
Cervical dysplasia or early invasive cervical cancer (Stage IA1) can be treated with loop electrosurgical excision procedure (LEEP) or cold knife cone (CKC).
ASCCP guidelines (www.asccp.org) should be used to triage abnormal cytology and histology.
Pathology (Refer to Table 2.4) [4]
Stage | Rate of pelvic lymph node metastases (%) | Rate of para aortic lymph node metastases (%) |
|---|---|---|
I | 15 | 6 |
II | 29 | 17 |
III | 47 | 30 |
There are four main routes of spread of cervical carcinoma:
Direct spread into the vaginal mucosa.
Spread into the myometrium, particularly with lesions originating in the endocervix.
Spread into the paracervical and parametrial lymphatics and then further (primarily: obturator, hypogastric, external iliac, and sacral nodes and secondarily: common iliac, inguinal, and para-aortic nodes) (Fig. 2.3) [4].
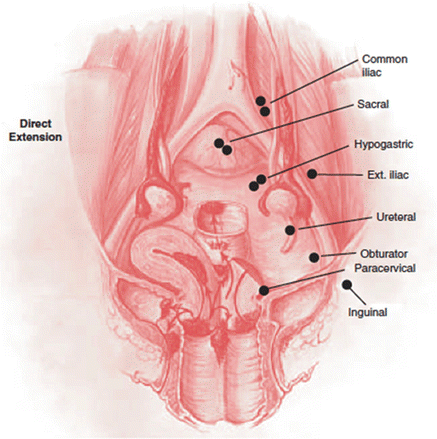
Fig. 2.3.
Patterns of lymphatic spread in cervical carcinoma [4]. Reprinted from Clinical Gynecologic Oncology, 7th Edition, Di Saia PJ, Creasman WT. Chapter 3 Invasive Cervical Cancer, Monk BJ, Tewari KS, Copyright 2007, with permission from Elsevier. Clinical gynecologic oncology by Di Saia PJ, Creasman WT. Reproduced with permission of Elsevier Mosby in the format reuse in a book/textbook via Copyright Clearance Center.
Direct extension into adjacent structures (parametria, bladder, bowel).
Adenocarcinomas arise from the endocervical mucous-producing glands and, because they originate within the endocervical canal, it takes longer until these tumors are clinically evident. This growth pattern results in the classic barrel-shaped cervix.
No difference in survival between cervical adenocarcinomas and squamous carcinomas after correction for stage (see Tables 2.5 and 2.6 [5]).
Stage
Surgery only (%)
Radiation only (%)
Surgery + radiation (%)
Ib1
94.5
80.1
83.6
Ib2
91.4
73.7
76.7
IIa
72.6
64.5
76.2
IIb
73.0
64.2
64.3
Table 2.6.
Histologic types of cervical cancer.
Pathology
Prevalence
Nonglandular
Squamous cell
65–85 %
Verrucous
Rare
Sarcomatoid
Rare
Glandular
Endocervical
10–25 %
Endometrioid
Rare
Clear cell
Rare
Mucinous
Rare
Serous
Rare
Adenoid cystic
Rare
Villoglandular
Rare
Other, mixed epithelial tumors
Adenosquamous
5 %
Glassy cell
Rare
Small cell
Rare
Nonepithelial tumors
Rare
Carcinosarcoma, leiomyosarcoma, endometrial stromal sarcoma, germ cell tumors, melanoma, lymphoma, neuroendocrine
1998 FIGO Annual Report of over 10,000 squamous cell carcinomas and 1,138 adenocarcinomas noted no difference in survival in Stage I cancers.
Staging
Cervical cancer is clinically staged based on (Table 2.7):
Table 2.7.
Cervical cancer staging according to the International Federation of Gynecology and Obstetrics (FIGO) revised in 2009.
FIGO Stage
Description
0
Carcinoma in situ
Ia1
Invasion of stroma <3 mm in depth and ≤7 mm in width
Ia2
Invasion of stroma >3 mm and ≤5 mm in depth and ≤7 mm in width
Ib1
Clinical lesions greater than Stage Ia but no greater than 4 cm
Ib2
Clinical lesions confined to the cervix that are greater than 4 cm
IIa
Involvement of the upper 2/3 vagina
IIb
Involvement of the parametria without sidewall involvement
IIIa
Extension to lower 1/3 vagina
IIIb
Extension to pelvic sidewall or hydronephrosis or non-functional kidney
IVa
Extension to bladder or rectum
IVB
Distant metastasis or disease beyond the pelvis
Exam.
CKC or LEEP.
Imaging—CXR, IVP, CT urogram, Barium enema.
Cystoscopy.
Proctosigmoidoscopy.
PET/CT Staging
In 2005 the Centers for Medicare and Medicaid Services approved coverage for FDG-PET for staging newly diagnosed and locally advanced cervical cancers and screening for cervical cancer recurrence.
Sensitivity of PET in detecting pelvic nodal metastases in patients with untreated cervical cancer = 80 %, sensitivity of CT = 48 % [6].
A 2007 meta-analysis of 41 studies concluded that PET/CT had the highest sensitivity (82 %) and specificity (95 %) for detection of positive nodes compared to CT (50 and 92 %) and MRI (56 and 91 %). PET positive nodes have been found to be a prognostic biomarker predicting treatment response, pelvic recurrence risk, and survival [6].
Genetics
There is no known genetic basis for cervical cancer.
Indication for and Modes of Treatment (Surgery/Chemotherapy/Radiation Therapy)
During the past several decades, staging definitions and treatment recommendations for cervical cancer have changed significantly (Table 2.8).
Table 2.8.
Treatment of cervical cancer by stage.
Stage
Standard treatment
Fertility preserving treatment
IA1, −LVSI
Extrafascial hysterectomy
Cervical cone biopsy
IA1, +LVSI
Extrafascial hysterectomy, +/− pelvic lymph node dissection
Cervical cone biopsy and laparoscopic pelvic lymph node dissection
IA2, occult IB1
Modified radical hysterectomy with pelvic lymph node dissection, +/− adjuvant therapy
Radical trachalectomy with pelvic lymph node dissection
IB1, IB2, IIA
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access