Recommendation
Level of evidencea
Insertion
Hand hygiene with soap and water or waterless alcohol gel
1A
Maximal sterile barrier precautions: cap, mask, sterile gown, sterile gloves, sterile full body drape
1A
Skin antisepsis with 2 % chlorhexidine
1A
Trained, competent provider to insert or oversee inexperienced personnel
1A
No prophylactic antibiotic
1B
Use of totally implanted device whenever possible due to decreased risk of infection
1B
Use of ultrasound-guided assistance not recommended with subclavian line placement
2A
Subclavian site is the preferred insertion site
2C
CVC placement can occur with ALL induction or be delayed
2C
Site care
Hand hygiene with soap and water or waterless alcohol gel
1A
Use either sterile transparent semipermeable dressing or sterile gauze and tape dressing (especially if diaphoretic or bleeding from site)
1A
Change sterile transparent semipermeable dressing every 5 days or when loose, wet, or soiled
1C
Change sterile gauze and tape dressing daily or if loose, wet, or soiled
1C
Chlorhexidine gluconate for exit site antisepsis
1A
Monitor site for evidence of infection
1C
No topical antibiotic at exit site
1B
Use of StatLock® for securement of PICC line
1B
Antiseptic-impregnated catheters are not routinely recommended
2C
Hub care
Scrub hub for 15 s with either 70 % isopropyl alcohol or 2 % chlorhexidine in 70 % isopropyl alcohol prior to every access
1B
Assessment
Daily assessment of site for evidence of infection
1C
Daily assessment of need for CVC
1A
Locking line
External catheters: daily (when not in use) and after intermittent use with heparinized saline (concentration/volume per institutional policy)
1C
Totally implanted device: monthly and after intermittent use with heparinized saline (concentration/volume according to institutional policy)
1C
Education
Dedicated CVC team to evaluate current literature
1B
Ongoing training for personnel of new policies, procedures, equipment
1B
Infection
Antibiotic ointment alone should not be used for exit site infections
1B
Catheter removal is indicated for tunnel infection
2A
Port catheter removal is indicated for pocket infection
1A
Occlusionb
Utilization of tPA dwell for CVC occlusion
2A
Low-dose systemic tPA if tPA dwell unsuccessful
2C
Imaging with compression US with Doppler and CT with venography if US nondiagnostic with a high level of suspicion
2A
17.2 Types of Central Venous Catheters
CVCs are divided into two categories: non-tunneled and tunneled. Each catheter type has specific line care needs, advantages, disadvantages and complications (Table 17.2). Selection of the optimal type of CVC for use in a specific disease or treatment protocol is not standardized. Factors to consider in catheter selection include the age and weight of the child, the length and intensity of therapy, frequency of blood sampling, anticipated supportive care interventions including transfusions, infusions and nutrition, level of patient activity, body image, and family ability to understand teaching and properly care for the line.
Table 17.2
Advantages and disadvantages of central venous catheters (CVCs)
Type of CVC | PICC | Broviac | Implanted port | Powerline |
|---|---|---|---|---|
Advantages | Immediate access | Immediate access | No required daily care (when not accessed) | Immediate access |
Bedside insertion | Painless blood sampling | Blood sampling (when accessed) | Blood sampling | |
Bedside removal | External portion repairable | Lower infection risk | Compatible with CT power injection | |
Blood sampling with ≥2.8 F Transfusions with ≥4.0 F | No restriction of activities | Use for stem cell pheresis | ||
Disadvantages | Frequent flushing | Surgical placement | Needle required for access | Surgical placement Daily flushing |
Sterile dressing changes | Daily flushing | |||
Phlebitis | Sterile dressing changes | Potential needle dislodgement | Sterile dressing changes | |
Increased infection risk | Bathing limitations | |||
Bathing limitations | No swimming | |||
No swimming | Potential for self-removal | |||
Potential for self-removal | External portion not repairable | |||
Impact on body image | Impact on body image |
17.2.1 Peripherally Inserted Central Catheter
A peripherally inserted central catheter (PICC) is the most frequently inserted non-tunneled CVC for short-term intravenous therapy and can remain in place for weeks to months. A PICC is the ideal central access device for oncology patients that present acutely ill and too unstable for anesthesia (e.g., mediastinal mass, airway compromise). Some institutions prefer a PICC during induction therapy for acute leukemia due to concern of an increased risk of catheter thrombosis associated with asparaginase therapy. The thin flexible silicone or polyurethane catheter is typically inserted into the basilic vein due to the ease of threading within this vessel. The catheter tip is placed into a large vessel, typically the distal superior vena cava (SVC), allowing for rapid dilution of medications and prevention of vessel damage from vesicants and hyperosmolar solutions (Pettit 2002; Burns 2005). Insertion complications include curling of the catheter, difficulty threading the catheter, multiple attempts to place the catheter, malposition or failure to insert the catheter and medial nerve damage (Pettit 2002; Burns 2005; Alomari and Falk 2006). Post-insertion imaging either with a chest radiograph or fluoroscopy should be obtained to document proper placement of the catheter tip.
Advantages of PICCs include the ability to insert at either the bedside or in interventional radiology, ability to remove at the bedside, decreased cost and decreased potential complications related to anesthesia or a surgical procedure. After insertion, the external portion of the catheter is measured and documented. Remeasurement with each dressing change ensures proper positioning. Smaller gauge (larger diameter) PICCs allow for blood sampling and red blood cell transfusions. The manufacturer’s recommendations and established institutional guidelines should be strictly followed. Disadvantages of a PICC include the need for sterile dressing changes, frequent flushing and a risk of phlebitis. The catheter lacks a cuff for stabilization creating an increased risk of dislodgement. Securing a PICC line is especially important in young or unstable patients. A sutureless securement device, StatLock®, is a housing unit that clips the PICC line suture wings into place with an adhesive patch, improving stabilization over tape. A prospective, randomized trial to evaluate the use of StatLock® versus sutures found an overall reduction in complications and specifically with a significant decrease in bloodstream infections (Yamamoto et al. 2002).
17.2.2 External Tunneled Central Venous Catheter
A Broviac catheter is the most commonly inserted external tunneled CVC in pediatrics (other external tunneled CVCs include Hickman, Groshong, Leonard, Hemocath and Powerline) and is available in either a single- or double-lumen system. Tunneled catheters are placed into a vein in the chest or neck and tunneled under the skin to secure for long-term use. Made of silicone or polyurethane, Broviac catheters are surgically inserted into either the internal jugular vein or subclavian vein with the tip placed into the distal SVC. The line is tunneled under the skin and exits on the anterior or lateral chest. A Dacron cuff stimulates tissue growth stabilizing the line in place while inhibiting bacterial migration. The cuff may be felt under the skin approximately 2 cm above the exit site.
A Powerline is a newer less frequently used cuffed polyurethane tunneled CVC available in single-, dual- or triple-lumen systems. In addition to the advantages of an external CVC, a Powerline is compatible with power injection of CT contrast (as needed for imaging) with a maximum flow rate of 5 mL/s and can be utilized for stem cell harvesting (BARD website 2014). Routine daily line care is similar to a Broviac though Powerlines are made of a firmer material and breaks in the external portion are not repairable, thereby requiring removal.
Advantages of an external CVC include easy access for delivery of intravenous therapies and painless blood sampling. In the event of tears or blockages, the external portion of the catheter is repairable with kits available from the manufacturer. Repair kits for each CVC size should be kept in stock at the institution. Disadvantages of an external CVC are requirement of surgical placement with anesthesia, increased risk of infection and thrombosis compared to implanted catheters, requirement for sterile dressing changes, daily heparin flushes, risk of kinking and breaking particularly with larger gauge (smaller diameter) sizes, limitations on activity (swimming and bathing), impact on body image, and potential for self-removal, especially with infants and toddlers. External CVCs carry a greater risk of infection than implanted ports as a result of the external site of the hubs and possibly secondary to the frequency of access for infusion, line flushing and blood sampling (Adler et al. 2006; Maki et al. 2006; Perdikaris et al. 2008).
17.2.3 Implanted Port
A port is a totally implanted tunneled CVC consisting of two sections, a plastic or titanium reservoir with a self-sealing rubber septum and a silicone or polyurethane catheter. The reservoir is placed in a surgically created pocket in the subcutaneous tissue below the clavicle and sutured to the fascia to ensure stabilization. The reservoir should not be placed directly beneath the surgical incision as accessing through the incision may lead to infection or skin breakdown (Baggott et al. 2002). The catheter is tunneled and inserted into either the internal jugular or subclavian vein with the tip in the distal SVC. The use of a non-coring Huber needle prolongs the life of the septum to approximately 2,000 punctures with a 22 gauge needle and 1,000 punctures if using a 19 gauge needle (BARD website 2014). If the port is to be used immediately, the surgeon may access the device in the operating room prior to development of postoperative swelling thereby preventing patient discomfort.
Advantages of an implanted port include decreased risk of infection, ease of blood sampling when accessed for use, no restrictions on swimming or bathing and no required daily care when not accessed (O’Grady et al. 2002; Adler et al. 2006). A disadvantage, particularly in small children, is the requirement for needle access through the skin. A lidocaine-based topical anesthetic cream (or ethyl chloride “cold” spray) is frequently used prior to access to decrease the discomfort of needle insertion. While accessed, site assessment is necessary as dislodgment may occur due to the patient’s activity or use of an inappropriate length Huber needle, potentially leading to infiltration or extravasation of infusions. Implanted ports may stay accessed for long periods of time, but it is recommended to reaccess with a fresh needle every 7 days. Mechanical complications, although quite rare, include damage to the port reservoir, separation of the catheter from the reservoir and erosion of the reservoir through the skin (Schulmeister 2010).
17.3 Catheter Insertion
Though rare, complications during insertion can arise and cause significant morbidity and include pneumothorax, hemothorax, chylothorax, malpositioning, arterial puncture and failure to place. Factors associated with complications include physician inexperience, multiple insertion attempts, prior catheterizations, patient anatomy, prior surgery or radiation in the area and a high body mass index (Mansfield et al. 1994; Lefrant et al. 2002; Kusminsky 2007). An insertion failure rate of up to 43 % and complication rate up to 24 % occurs with ≥3 insertion attempts leading to a recommendation of limiting each operator to a maximum of two unsuccessful attempts (Mansfield et al. 1994; Eisen et al. 2006). The definition of an “attempt” varies among studies ranging from one puncture to multiple punctures by one operator at one site making comparisons difficult (Eisen et al. 2006; Balls et al. 2010).
Several studies have been completed evaluating the advantage of using real-time ultrasound-guided assistance (UGA) rather than the anatomic landmark technique for placement of a CVC (Augoustides and Cheung 2009; Pittiruti et al. 2009; Balls et al. 2010). A meta-analysis by Randolph et al. (1996) concluded that this technique led to an improved insertion success rate and a decrease in complications in both internal jugular and subclavian vein insertions. McGee and Gould (2003) found that UGA is effective in catheterization of the internal jugular vein with a decreased incidence of mechanical complications and placement failure. However, they found no benefit using this technique with subclavian vein insertions as the clavicle lies directly over the vessel, impeding visualization. In their studies, Mansfield et al. (1994) and Troianos et al. (2011) reached a similar conclusion. A retrospective observational study by Balls et al. (2010) assessed 1,222 CVC placement attempts concluding that the use of UGA did not improve the success of placement on the first attempt but overall saw a reduced number of total attempts. Further study is required to determine whether the routine use of UGA is feasible due to the high cost of equipment, required personnel training and equipment maintenance (Randolph et al. 1996).
Catheter insertion in the subclavian vein carries a higher risk of pneumothorax, malpositioning and failure to place compared to internal jugular insertion, while internal jugular catheterization is associated with a higher incidence of arterial puncture and hematoma (McGee and Gould 2003; Eisen et al. 2006). Although the subclavian vein carries the greater risk of insertion complications, it remains the preferred approach due to a lower rate of infection noted in some studies (McGee and Gould 2003). A prospective, observational study by Deshpande et al. (2005) found no difference in CVC infection rates for subclavian, internal jugular or femoral vein insertion sites in adult patients. The 2011 Centers for Disease Control (CDC) Guidelines for the Prevention of Intravascular Catheter-Related Infections declined to make a recommendation for the preferred CVC insertion site leaving the issue unresolved (O’Grady et al. 2011).
Children with acute lymphoblastic leukemia (ALL) often present with neutropenia and thrombocytopenia theoretically putting them at increased risk for complications with CVC placement. However, two separate studies evaluating 172 and 98 children, respectively, found no increased rate of complication with early CVC placement in newly diagnosed ALL patients (Handrup et al. 2010; Gonzalez et al. 2012). Platelet thresholds for CVC placement are undefined (see Chap. 2). Handrup et al. (2010) also concluded that the nonelective removal rate was similar between early and later placed CVCs. A retrospective analysis of 362 patients with ALL assessed complication rates between timing of insertion (early, ≤day 15 of induction, vs. late, >day 15 of induction) and type of CVC (ports vs. external CVCs) and found that early placement was associated with an increased risk of a positive blood culture and external CVCs were associated with an increased risk of positive blood cultures, thrombotic complications, and early removal (McLean et al. 2005). Due to the conflicting evidence, institutions providing initial care of newly diagnosed oncology patients must decide on the benefit of CVC placement timing, with ongoing monitoring for early complications and of line care in this setting.
17.4 Infection
Infection remains the major complication of an indwelling CVC, with bloodstream infection causing the most significant risk of morbidity and mortality. Terms used to describe intravascular catheter-related infection are confusing with central line-associated bloodstream infection (CLABSI) and catheter-related bloodstream infection (CRBSI) often used interchangeably. CLABSI is defined as an infection occurring in the patient with a CVC and not related to an infection at another site and is the term used by the CDC National Healthcare Safety Network (NHSN). CRBSI is a clinical definition requiring specific laboratory testing, quantitative blood cultures, differential time to positivity or culture of a segment of the removed catheter (O’Grady et al. 2011). Common organisms causing CLABSI include Staphylococcus epidermis, Staphylococcus aureus, Enterococcus faecalis, Klebsiella pneumoniae, Pseudomonas aeruginosa and Candida albicans. See Chaps. 1 and 14 for prevention, recognition and treatment of suspected infection or sepsis.
Most CVC-related infections are thought to occur by one of two methods: colonization at the exit site with pathogen migration along the external catheter surface or hub contamination leading to intraluminal colonization with spread into the circulation (McGee and Gould 2003). Within hours of CVC placement, a protein-rich sheath begins developing, covering the external and internal surfaces of the catheter. The protein sheath allows adherence of microbes which then produce a slimy substance (biofilm) becoming embedded in the matrix (Raad et al. 1993). Pathogens within a biofilm behave differently with an increased rate of reproduction and a greater resistance to antimicrobial therapy (Raad et al. 1993; Donlan 2011).
Antiseptic-impregnated catheters (AIC) coated with either chlorhexidine and silver sulfadiazine (CSS) or minocycline-rifampin (MR) have been studied in an effort to determine their effectiveness in decreasing the rate of CLABSI. Randomized clinical trials have generally not shown these catheters to be beneficial (McGee and Gould 2003). In a randomized clinical trial evaluating 232 catheters inserted in 180 critically ill hospitalized adult patients in use <10 days, there was no significant difference in the rates of colonization between antiseptic-impregnated and non-impregnated catheters (Theaker et al. 2002). Separate meta-analyses reviewing randomized controlled trials comparing AICs and non-AICs with a median insertion duration of 7–12 days concluded the efficacy of CSS catheters to be <2 weeks with MR catheters being effective somewhat longer (Mermel 2000; Walder et al. 2002). The results of numerous studies are difficult to compare with no type of catheter showing a definitive advantage. The CDC recommends that institutions develop strategies to provide education of personnel who insert and maintain catheters, with use of maximal sterile barrier precautions (i.e., cap, mask, sterile gown, sterile gloves and a sterile full body drape for line insertion) and skin antisepsis with >0.5 % chlorhexidine with alcohol for insertion. The use of antimicrobial-coated catheters is recommended at institutions where implementation of these CDC strategies fails to decrease CLABSI rates (O’Grady et al. 2011). Maki et al. (2006) determined that institutions with a baseline CLABSI rate of >2 % would benefit from use of AICs as this was the threshold at which AICs would decrease overall costs.
17.4.1 Exit Site Infection
An exit site infection is characterized by the presence of erythema, tenderness, induration or drainage within 2 cm of the catheter exit site, without signs or symptoms of systemic infection (O’Grady et al. 2011). Culture of the site should be obtained. Though not evidence-based, generally Gram-positive infections may be treated with oral antibiotics, while broad-spectrum parenteral antibiotics are indicated for Gram-negative organisms and for children with neutropenia (Baggott et al. 2002). Once the organism is identified, antibiotic therapy should be tailored to sensitivities. An exit site infection due to waterborne organisms, such as Pseudomonas spp., or fungus generally requires catheter removal as these organisms are notoriously difficult to clear. Antibiotic ointment alone should not be used at the exit site as this significantly increases the risk of Candida spp. infection and promotes antibiotic resistance (Zakrzewska-Bode et al. 1995; O’Grady et al. 2011).
17.4.2 Tunnel Infection
A tunnel infection is defined as tenderness, erythema, drainage or site induration >2 cm from the catheter exit site along the subcutaneous tract in the absence of concomitant CLABSI (O’Grady et al. 2011). Blood cultures from the CVC and skin cultures should be obtained. Catheter removal is indicated and parenteral antibiotics tailored to sensitivities of the cultured organism are given for 7–10 days (Mermel et al. 2009). A PICC may be placed to complete the recommended course of antibiotics.
17.4.3 Pocket Infection
A pocket infection involves erythema, tenderness and swelling over the site of an implanted port with purulent fluid noted in the subcutaneous tissue. Drainage or necrosis of the overlying skin may be present (O’Grady et al. 2011). Drainage should be obtained and cultured. Removal of the port is indicated with debridement, if necessary. A course of parenteral antibiotics is essential with medication tailored to the sensitivity of the infecting organism (O’Grady et al. 2002). Prior to insertion of another CVC, the wound should be healed, the course of antibiotics completed and the child should have defervesced. Consideration may be given to placement of a PICC should a CVC be needed to complete therapy.
17.4.4 Prevention of Infection
A CVC bundle is a set of evidence-based care practices implemented to decrease the risk of infection due to the presence of a CVC. Components include hand hygiene, selection of the optimal insertion site, use of maximal barrier technique, chlorhexidine skin antisepsis and prompt removal of the catheter when it is no longer needed (O’Grady et al. 2011). Development of institutional guidelines and ongoing staff education are essential in decreasing infection rates (O’Grady et al. 2011). Each institution’s infection control department is instrumental in tracking rates of CLABSI. Cooperation with the hematology/oncology service is imperative for ongoing evaluation with changes to institutional practices as indicated. A local expert on CVC care and management and infection control will enhance education, monitor adherence to policy, follow rates of infection and evaluate the current literature (Teichgraber et al. 2011). Placement of the institution’s hand hygiene guidelines in patient care areas is a great reminder for practitioners, patients and family members. Further discussion of prevention of CVC line infection is detailed in Chap. 14.
17.5 Occlusions
A functioning CVC is a catheter that flushes easily, infuses without difficulty and has brisk blood return (Baskin et al. 2009). Occlusion is the most common noninfectious complication of CVCs with an occurrence rate of 25 % and resulting in delays in the administration of chemotherapy and supportive care (Stephens et al. 1995). Rapid assessment is needed to determine both the cause of the obstruction and the appropriate interventions. Causes of CVC occlusion include mechanical complications, drug precipitate or lipid residue and thrombosis. Each of these problems can result in either partial or complete obstruction of the catheter. A partial occlusion allows for fluid infusion but either a sluggish or complete inability to withdraw blood (ball-valve effect). A complete occlusion allows neither fluid infusion nor blood withdrawal. An unusual problem may occur with implanted ports allowing blood withdrawal but not fluid administration due to a thrombus inside the reservoir at the outlet port (a reverse ball-valve effect).
17.5.1 Mechanical Occlusion
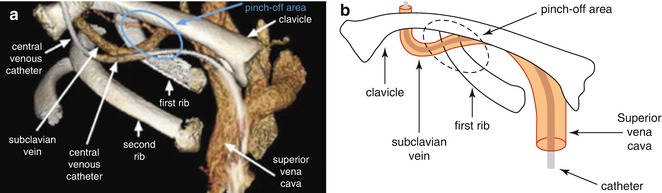
The cause of a mechanical obstruction may be as simple as a closed clamp, a kink in the external portion of the line or an exit site suture that is too tight, all of which are easily corrected after careful inspection and manipulation. An improperly inserted Huber needle is corrected by re-accessing the implanted port. A “pinch-off” syndrome (Fig. 17.1) can occur with catheter compression between the clavicle and first rib at a reported 1 % incidence rate. This complication is associated with insertion into the subclavian vein via an infraclavicular approach (Fazeny-Dorner et al. 2003; Baskin et al. 2009). Rolling the shoulder forward or raising the arm on the opposite side may allow blood withdrawal. Over time, compression may lead to fracture of the catheter. A chest radiograph or fluoroscopic examination aids in diagnosis with immediate removal indicated if confirmed. A rare but life-threatening complication is fragmentation of a distal portion of the catheter with migration to the heart or pulmonary artery. Symptoms include shoulder and chest pain, palpitations and arrhythmias (Nace and Ingle 1993). Patients may be asymptomatic except for pain with attempted infusion (Dillon and Foglia 2006). Retrieval of the embolized catheter is generally accomplished by interventional radiology or cardiology using loop snares, baskets or guide wires (Sagar and Lederer 2004).