Painful diabetic neuropathy
Painful distal symmetrical polyneuropathy (painful diabetic neuropathy) affects about 20% of people with diabetes and is defined as “pain as a direct consequence of abnormalities in the peripheral somatosensory system in people with diabetes” . It is associated with a high healthcare burden and reduced productivity . In the United Kingdom, the average annual healthcare cost per patient with painful diabetic neuropathy is £2511 . A cross-sectional study, also in the United Kingdom, revealed that approximately 60% of working patients with painful diabetic neuropathy felt being less productive at work . The pain experienced by patients can considerably interfere with their daily activities, work, mood, and sleep, and is associated with a reduced quality of life . Patients with painful diabetic neuropathy are also more likely to suffer from depression and emotional distress . Pain is a complex multidimensional process, which not only affects sensory but also emotional/cognitive processing. For example, chronic pain patients are defined as “difficult patients” in that they often have neuropsychological changes that include changes in affect and motivation or changes in cognition, all of which rarely predate their pain condition. Chronic pain may also arise after the onset of depression, even in patients without a prior history of pain or depression. Taken together, these clinical insights suggest an integral role of the central nervous system (CNS) in the pathogenesis and maintenance of the chronic pain state.
The management of painful diabetic neuropathy generally involves optimization of glycaemic control, modification of cardiovascular risk factors, and symptomatic relief . Currently, the mainstay of symptomatic treatment for diabetic neuropathy is pharmacotherapy and this is covered in greater detail in other chapters. It is clear that the CNS is the main site of action of many of the agents used to manage neuropathic pain, thus also hints at its important role in painful diabetic neuropathy.
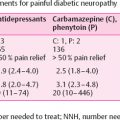
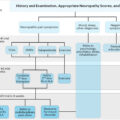
Despite recent advances and considerable research, treatment for neuropathic pain is largely ineffective. A meta -analysis that evaluated the medical management of neuropathic pain found overall only modest outcomes, with the number needed to treat to achieve 50% pain relief ranging from 4 to 10 . In addition, Phase III trials of disease-modifying therapies such as α-lipoic acid and benfotiamine have largely shown equivocal results . At present, there is no pathogenetic treatment found to provide sufficient pain relief or prevent the development of neuropathic pain in diabetes . One possible reason for this is because the pathogenesis of painful diabetic neuropathy is not fully understood. More robust and better designed studies are also required to further assess the benefits of future novel therapies . There is an emerging theory, which postulates that the wide variability in treatment response may in part be due to an underlying heterogeneity in clinical pain phenotypes . Using quantitative sensory testing (QST), distal symmetrical painful neuropathy (DSP) patients can be broadly subdivided into two phenotypes: “irritable” (IR, with relatively preserved sensory function associated with thermal and/or mechanical hyperalgesia) and “nonirritable” (NIR, dominated by thermal and mechanical sensory loss) . Subsequent studies appear to suggest that some treatments are seemingly more effective in those with the IR compared to the NIR nociceptor phenotype (for a review, see and for examples, see ). Consequently, pain phenotyping may, in the future, become important in guiding individual patients’ treatment although the exact approach is heavily under debate. This chapter will focus on the role for CNS imaging in phenotyping patients with painful Diabetic peripheral neuropathy (DPN) for personalized therapy.
There is growing evidence suggesting that maladaptive alterations in the CNS, not just the peripheral nervous system, play a role in the generation and maintenance of neuropathic pain . The CNS not only detects and localizes pain, but is also involved in the perception of pain or nociceptive inputs . Changes in the CNS such as descending inhibition of pain, neurotransmitter imbalance, central sensitization, and cortical reorganization may be implicated in the pathogenesis of neuropathic pain in diabetes . The structural and functional changes in the CNS in painful conditions, including painful diabetic neuropathy, have also been supported by various imaging studies .
Central nervous system changes in response to peripheral nerve injury and how these changes result in chronic pain in diabetes
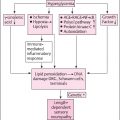
Neuropathic pain is a combination of sensory loss and maladaptive changes in the peripheral and CNSs ( Fig. 6.1A,B ). The molecular/ion channel alterations have been covered in greater detail in previous chapters. The changes in the ion channels and ectopic activity at the periphery lead to enhanced synaptic transmission within the dorsal horn of the spinal cord and amplification of nociceptive information, a process known as central sensitization . Central sensitization and the altered balance between inhibitory interneurons and the descending pain modulatory system can either facilitate or inhibit the transmission of nociceptive information at spinal levels . The altered balance within these systems (gain of excitation or loss of inhibition) is believed to also contribute to the development of neuropathic pain. Conditioned pain modulation is a technique to test for the integrity of the descending pain modulatory system; a painful conditioning stimulus applied to one body site reduces pain caused by a different stimulus in another body site. It is found to be impaired in patients with various pain syndromes, including painful diabetic neuropathy . Variability in the pain modulation mechanisms among individuals may partly explain why some patients experience more debilitating pain than others and why some patients respond to pharmacological and nonpharmacological therapies but not others .
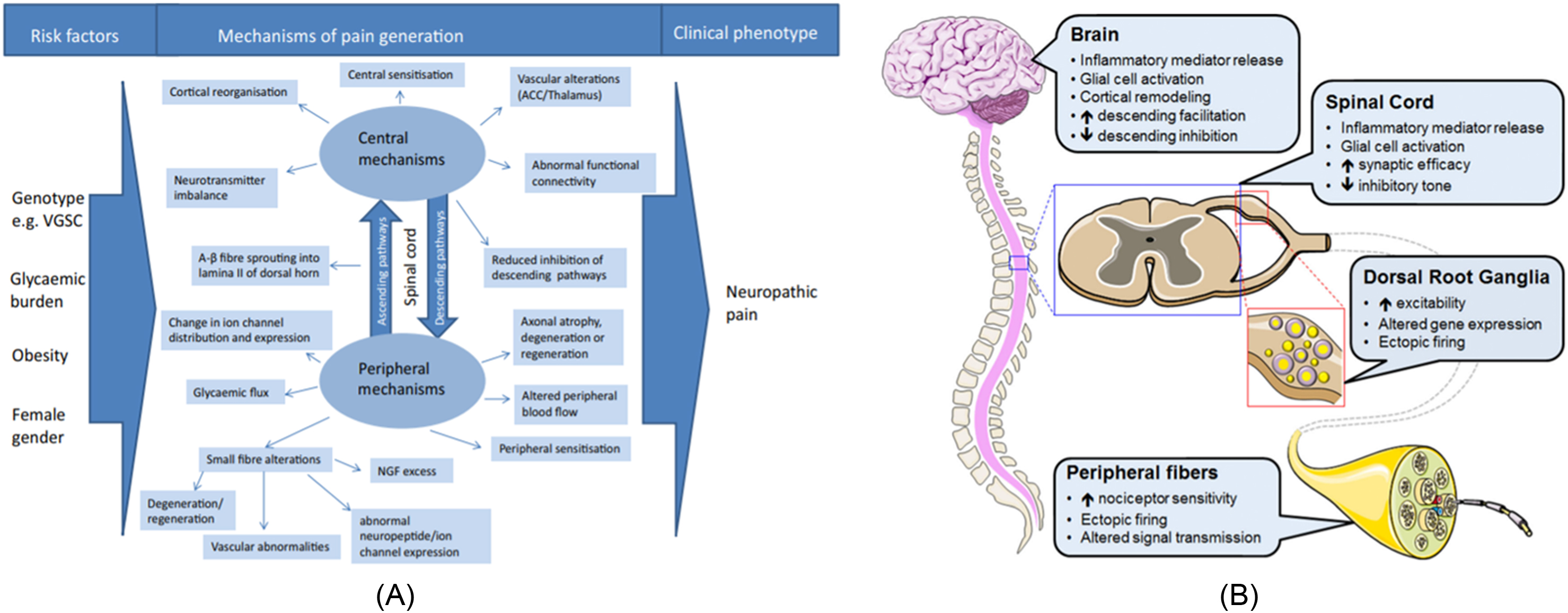
Changes in the spinal cord
In recent years, there have been a number of neuroimaging studies that have exploited advances in multimodal magnetic resonance imaging (MRI) to gain new insights into CNS involvement in diabetic neuropathy. Eaton et al. revealed significant reduction in the spinal cord cross-sectional area in the cervical and upper thoracic regions in patients with advanced diabetic neuropathy compared to nondiabetic controls . A subsequent, larger study confirmed these findings but also revealed that spinal cord atrophy was present in subjects with early, subclinical diabetic neuropathy. Moreover, subjects with hereditary sensorimotor neuropathy, an inherited neuropathy localized to the peripheral nerves, had preserved cord cross-sectional area. This argues against the “dying-back” phenomenon , that is, peripheral nerve injury leading to subsequent cord atrophy in diabetic neuropathy rather it suggests that diabetes causes a generalized, concomitant insult to both the peripheral and CNSs .
Changes in the brain—structural and functional
Further investigations into CNS involvement in diabetic neuropathy have demonstrated structural changes in the brain. Patients with diabetic neuropathy displayed abnormalities in the peripheral (pre- and postcentral gyrus) and deep gray matter (GM) nuclei (caudate, putamen, medial pallidum, thalamus, and ventral nuclear) in regions involved with somatosensory/nociception perception. Moreover, there were alterations in the white matter (WM) tracts (spinothalamic tract, corticospinal tract, and thalamocortical projecting tracts) indicative of the involvement of somatosensory, motor, and pain-related pathways in diabetic neuropathy . Specific alterations in patients with painful diabetic neuropathy have been reported in the cingulate cortex, insular cortex, prefrontal lobe, thalamus, periaqueductal WM, and external capsule . The involvement of the anterior cingulate cortex (ACC) and prefrontal cortex has been implicated in the unpleasant perception associated with allodynia (pain due to a stimulus that normally does not provoke pain) . Both patients with painful and painless diabetic neuropathy were also found to have reduced GM volume localized to regions involved in somatosensory perception compared to control patients with diabetes and no diabetic neuropathy and healthy volunteers . Using proton MR spectroscopy to examine neurochemical alterations in the thalamus, patients with painless diabetic neuropathy demonstrated abnormal thalamic neuronal integrity compared to those with painful diabetic neuropathy. This was supported by structural MRI that demonstrated that thalamic GM volume was reduced in subjects with painless diabetic neuropathy but not painful diabetic neuropathy, suggesting that preservation of thalamic neuronal integrity may play a key role in the perception of pain in diabetic neuropathy . The thalamus acts as a relay for the transmission of nociceptive information from the periphery to other areas of the brain . Animal models of painful diabetic neuropathy have demonstrated increased spontaneous activity from the thalamic nuclei . Magnetic resonance perfusion imaging of the brain has shown increased thalamic vascularity in patients with painful diabetic neuropathy . These findings imply the possible involvement of the thalamus in central amplification of somatosensory signals . Similar changes have also been observed in the ACC . Patients with painful diabetic neuropathy displayed increased cerebral blood flow in the ACC, suggesting that ACC activation is implicated in the development of central sensitization in response to peripheral neuropathic pain . In addition, patients with painful diabetic neuropathy have altered functional connectivity in the ventrolateral Periaqueductal grey (PAG), which correlates with their pain intensity and cerebral blood flow in response to tonic heat stimulation . This supports the idea that abnormalities in the PAG may result not only in reduced inhibition, but also in facilitation of pain . Collectively, these findings, supported by numerous studies in other chronic pain conditions, demonstrate dynamic neuronal changes that have profound effects on the brain in patients with diabetic neuropathy. More recently, it has been demonstrated how these structural and functional changes are related to painful DPN clinical phenotypes.
Functional magnetic resonance imaging (fMRI) is a modality of MRI, which measures brain activity by detecting changes in blood flow [bold oxygen level dependent (BOLD) signal] that is coupled with neuronal activation, that is, when an area of the brain is in use, blood flow to that region also increases and this is assessed using fMRI. Using this modality of MRI, several studies have identified in vivo the neurological signature for both physical and neuropathic pain (capsaicin model). The brain regions activated in response to a nociceptive stimulus can be divided into somatic region responsible for the localization of pain and coding intensity such as the ventrolateral thalamus, somatosensory cortex, dorsal posterior insula, and the emotional/affective regions responsible for emotional pain processing such as the anterior insula, dorsal lateral prefrontal cortex, and ACC .
Recent studies
A case-controlled, multimodal MRI study of carefully phenotyped patients demonstrated a pathophysiological relationship between anatomical and functional changes in the brain and sensory phenotypes. Subjects with insensate painful diabetic neuropathy (i.e., painful “painless” diabetic neuropathy) had the lowest S1 cortical thickness and greatest S1 cortical functional reorganization with expansion of the area representing pain in the lower limb region to include face and lips regions compared to painful diabetic neuropathy subjects with relatively preserved sensation. Furthermore, the extent to which S1 cortical structure and function are altered was related to the severity of neuropathy and the magnitude of self-reported pain. These data suggest a dynamic plasticity of the brain in diabetic neuropathy, driven by the neuropathic process and may ultimately determine an individual’s clinical pain phenotype .
Over the last decade, resting-state fMRI (RS-fMRI)—a quick and simple, noninvasive technique—has become an increasingly appealing method to examine spontaneous brain activity in individuals without relying on experimental external stimulation tasks. During a typical RS-fMRI examination, the hemodynamic response to spontaneous neuronal activity (BOLD) signal is acquired while subjects are instructed to simply rest in the MRI scanner . Data acquired are used in brain mapping to evaluate regional interactions or functional connectivity that occur in a resting state. Most studies use a machine learning approach to identify patterns of functional connectivity that differentiate patients from controls. RS-fMRI experiments in painful DPN have reported greater thalamic-insula functional connectivity and decreased thalamic-somatosensory cortical functional connectivity in patients with the IR nociceptor phenotype compared to the NIR nociceptor phenotype. There was a significant positive correlation between thalamic-insula functional connectivity with self-reported pain scores . Conversely, there was a greater reduction in thalamic-somatosensory cortical functional connectivity in those with more severe neuropathy. This demonstrates how RS-MRI measures of functional connectivity relate to both the somatic and nonsomatic assessments of painful DPN. Using a machine learning approach to integrate anatomical and functional connectivity, data achieved an accuracy of 92% and sensitivity of 90% indicating good overall performance . Multimodal MRI combining structural and RS-fMRI has also been used to predict treatment response in painful DPN. Responders to intravenous lidocaine treatment have significantly greater S1 cortical volume and greater functional connectivity between the insular cortex and corticolimbic system compared to nonresponders . The insula cortex plays a pivotal role in processing the emotion and cognitive dimensions of the chronic pain experience. The corticolimbic circuits have also long been implicated in reward, decision making, and fear learning. Hence, these findings suggest that this network may have a role in determining treatment response in painful DPN. Using advanced multimodal MR neuroimaging, a number of studies have demonstrated alterations in pain-processing brain regions that relate to clinical pain phenotype, treatment response, and behavioral/psychological factors impacted by pain. Taken together, these assessments could serve as a possible Central Pain Signature for painful DPN. The challenge now is to apply this potential pain biomarker at an individual level in order to demonstrate clinical utility. To this end, the application of a machine learning approach to classify individual patients into different clinical pain phenotypes using brain imaging features taken from a quick, 6-minute RS-fMRI scan is appealing.
Maladaptive responses
The perception of pain depends on the complex interaction between cognitive, emotional, socio-cultural, and physical factors . Emotions and cognitive factors such as attention and memory influence how patients perceive pain; negative emotional states can cause increased pain whereas positive states result in reduced pain . Depression and anxiety are frequently present in patients with neuropathic pain and are associated with catastrophizing, which is characterized by feeling of helplessness, inability to cope with the pain and propensity to exaggerate the threat value of pain . This can in turn lead to maladaptive behavior such as avoidance of activities, which promotes disability and persistence of pain . The significance of this is also evident in the therapeutic context, as a patient’s enhanced or diminished response to pharmacotherapy may be influenced by his or her expectation, emotional state, and attention . It also highlights the important role of the CNS in painful diabetic neuropathy.
In conclusion, neuronal damage leads to maladaptive responses in the nociceptive pathway and the nervous system, which is the driver of persistent and chronic pain . QST profiles have revealed three distinct phenotypes in patients suffering from neuropathic pain, namely sensory loss, thermal hyperalgesia, and mechanical hyperalgesia, which reflect the underlying pathophysiological mechanisms . For example, peripheral sensitization is the predominant mechanism in thermal hyperalgesia while central sensitization is mainly involved in mechanical hyperalgesia . Patients with diabetic neuropathy also manifest these phenotypes, with sensory loss being the most common (83%), followed by thermal hyperalgesia (75%), and mechanical hyperalgesia (34%) . Further stratification of patients based on their phenotypes and key pathophysiological driver(s) is necessary to provide individualized treatment to optimize outcomes . More research is required to determine the causality of CNS changes in patients with diabetic neuropathy to help direct management as changes in the architecture of the brain and spinal cord, especially early in the disease process may imply irreversibility and may be associated with poorer outcomes . A better understanding of the underlying mechanisms for maladaptive plasticity may help to identify specific therapeutic targets to prevent the development of neuropathic pain and to normalize function in patients with established neuropathic pain .
Clinical implications
As highlighted in this chapter, brain imaging has the potential to fast-track the development of new therapeutic interventions and alter the management approach for painful diabetic neuropathy. Preliminary findings from the studies conducted to date suggest their utility as diagnostic, prognostic, and pharmacodynamic biomarkers that should be carefully considered for possible inclusion in clinical trials of pain treatments. There are of course limitations and the usefulness of biomarkers remains a controversial topic. Focused research is now needed to standardize the application of neuroimaging biomarkers to address the heterogeneity in their application in terms of methodological approach, outcomes examined, and time points used for assessments. The direct clinical application of brain imaging in practice will also be dependent on a robust health economic analysis of screening and interventions with study designs that allow relevant and appropriate cost comparisons. A crucial element here is the patient’s perspective on the usefulness, acceptance, and feasibility of these novel imaging technologies in the provision of future services. We focused on the application of CNS neuroimaging on people with painful diabetic neuropathy throughout the chapter as the vast majority of studies performed were in this group. The clinical implications of CNS involvement in patients with painless diabetic neuropathy, on the other hand, remain much to be understood—for example, the association and possible mechanisms of neuropathy on cognitive decline and the indifference observed in some patients with recurrent diabetic foot ulcers when it comes to observing preventative foot care practices despite of the increased risk of major amputations.
Future direction
Future research has a significant role in addressing the interrelationships between the peripheral and CNS in painful diabetic neuropathy. Future research directions can be divided into three key areas: mechanistic or pathophysiological, performing clinical trials relevant to an individual with painful diabetic neuropathy and influencing federal agencies/commissioners of diabetes and pain services. Brain imaging techniques such as fMRI, positron emission tomography (PET), and proton magnetic resonance spectroscopy ( 1 H-MRS) provide neurochemical, structural, or functional information on the processing and modulation of nociceptive inputs in the brain, which result in the perception of pain . Table 6.1 summarizes some of the benefits and limitations of these imaging modalities . Neuroimaging studies have shown differences in the areas of brain activated in acute pain and chronic pain as well as differences in functional connectivity between affected patients and healthy controls, thus providing diagnostic clues of the brain patterns in pain conditions and the underlying mechanisms involved in the generation, maintenance, and exacerbation of chronic pain . As a prognostic tool, brain imaging can be used to identify individuals who are more “vulnerable” or “resilient” against developing persistent pain after being exposed to factors that can trigger the onset of chronic pain . For example, increased functional connectivity of the nucleus accumbens with prefrontal cortex predicts transition from acute pain to chronic pain in subjects with subacute back pain . Brain imaging can be used to predict treatment response, hence may enable stratification of participants in clinical trials . fMRI data derived from visual stimulation have shown a greater than 80% accuracy in distinguishing whether a patient with fibromyalgia was administered pregabalin or placebo . Brain imaging can be a useful diagnostic, prognostic, and predictive tool and its use as a potential biomarker in chronic pain clinical trials requires further research focussing on standardization of outcomes, validation, reproducibility, and evaluation of outcomes .