Fig. 13.1
Algorithm to diagnose and to treat empirically/preemptively CNS infections in hematology patients
Lumbar puncture and cerebrospinal fluid (CSF) analysis should follow the algorithm in Fig. 13.1 and are generally performed after neuroimaging in the absence of contraindications such as transfusion-refractory thrombocytopenia or critically increased intracranial pressure. Routine CSF analyses should be performed according to published guidelines and include the differential cell count, determination of the glucose and protein concentration, Gram staining, and bacterial cultures [13]. Additional CSF examinations such as specific PCR assays or India ink smears are useful in some situations (see below, also Table 13.1). The aim is to make an unambiguous diagnosis of CNS infections, but this cannot always be achieved by the combination of clinical symptoms, CSF analyses, and neuroimaging. In patients with focal lesions, biopsy (e.g., stereotactic) and/or neurosurgical interventions (e.g., abscess drainage or resection of lesions) should therefore be considered as modalities for identifying causative organisms and determining their in vitro susceptibilities in individual patients [72].
Table 13.1
Recommended examinations of biopsy material or CSF to diagnose CNS infections caused by fungi, Toxoplasma gondii, or viruses in hematology patients
Causative organism | Recommended diagnostic examinations | |
|---|---|---|
Fungi | Aspergillus spp. | Biopsy material |
Fungal cultures and direct microscopy | ||
CSF (Aspergillus meningitis) | ||
Detection of Aspergillus DNA (PCR) or galactomannan (ELISA) (if feasible) | ||
Candida spp. | Biopsy material | |
Fungal cultures and direct microscopy | ||
CSF | ||
Fungal cultures (sensitivity about 40–80 %) and direct microscopy (sensitivity about 40 %)a | ||
Enzyme immunoassay formats for rapid detection of species-specific amplicons and the use of real-time PCR (if feasible) | ||
Detection of Candida antigen mannan (if feasible) | ||
Cryptococcus neoformans | Biopsy material | |
Direct microscopy (e.g., after PAS or H&E staining) | ||
CSF | ||
Fungal cultures (sensitivity and specificity up to 90–100 %) | ||
India ink smear examination (sensitivity 50–94 %), latex antigen test (sensitivity and specificity up to 100 %), and PCR (sensitivity and specificity nearly 100 %) | ||
Zygomycetes | Biopsy material | |
Fungal cultures and direct microscopy | ||
Molecular-based tests (if feasible) | ||
Parasites | Toxoplasma gondii | Biopsy material or CSF |
Demonstration of tachyzoites and/or cysts after Wright-Giemsa and/or immunoperoxidase staining (can also be done after mouse inoculation or tissue cultures) | ||
CSF | ||
PCR (sensitivity about 50–90 %; specificity 90–100 %) | ||
Serological investigations (ELISA is more sensitive than latex agglutination test) | ||
Virusesb | HHV-6 | CSF |
PCR (sensitivity above 95 %, PCR in CSF can be positive without evidence of HHV-6 encephalitis in brain biopsy or autopsy specimens) | ||
EBV (virus encephalitis) | CSF | |
PCR (can be combined with serological techniques) | ||
HSV | CSF | |
PCR (sensitivity and specificity 90–100 % compared to brain biopsy) | ||
Immunoassays for intrathecal anti-HSV antibody production | ||
CMV | CSF | |
PCR (sensitivity 82–100 %; specificity 86–100 %; PCR results may be confirmed by cultures) | ||
VZV | CSF | |
PCR (sensitivity 80–95 %, specificity >95 %; copy number in real-time PCR may correlate with the clinical severity of encephalitis) | ||
Sensitivity of PCR might be enhanced by serological tests (e.g., detection of CSF VZV IgM) | ||
JC virus (PML) | Biopsy | |
Required for definitive diagnosis: demonstration of the typical histopathologic triad (demyelination, bizarre astrocytes, and enlarged oligodendroglial nuclei), might be combined with tissue JC virus PCR | ||
CSF | ||
PCR (sensitivity 75–100 %), might be false positive (e.g., due to JC virus viremia in healthy individuals) | ||
13.1.5 Treatment
Efforts should always be directed towards identifying a causative organism and testing its susceptibility prior to the initiation of anti-infective drug therapy. Empirical or preemptive anti-infective drug therapy should typically be started immediately, as long as the causative pathogen has not yet been identified, since treatment delay may increase the mortality rate [65, 66]. Figure 13.1 specifies empirical or preemptive anti-infective drug regimens for different suspected organisms, while Table 13.2 summarizes recommendations for anti-infective drug therapy of defined CNS infections, including categories of evidence [28, 53].
Table 13.2
Anti-infective drug therapy recommended for selected CNS infections in hematology patients
Causative organism | Recommended therapy | |
|---|---|---|
Fungi | Aspergillus spp. | Voriconazole (6 mg/kg q12h for the first 24 h, then 4 mg/kg q12h) [A II] |
Alternative | ||
Liposomal amphotericin B (3–5 mg/kg/d) or ABLC (5 mg/kg/d) [B III] | ||
Candida spp. | Voriconazole (6 mg/kg q12h for the first 24 h, then 4 mg/kg q12h) [BIII] | |
Alternative | ||
Liposomal amphotericin B (3–4 mg/kg/d) or ABLC (5 mg/kg/d) [B III] | ||
Cryptococcus neoformans | Liposomal amphotericin B (3–4 mg/kg/d) + 5-fluorocytosine (25 mg/kg q6h) [A III]a | |
Alternative | ||
Liposomal amphotericin B (3–5 mg/kg/d) [B I] or ABLC (5 mg/kg/d) [B III] or | ||
Amphotericin B deoxycholate (0.7 mg/kg/d) + 5-fluorocytosine (25 mg/kg q6h) [B I] or | ||
Voriconazole (6 mg/kg q12h for the first 24 h, then 4 mg/kg q12h) [B III] | ||
Zygomycetes | Liposomal amphotericin B (3–5 mg/kg/d) [A II] or ABLC (5 mg/kg/d) [B III] | |
Alternative | ||
Posaconazole (orally, 400 mg q12h or 200 mg q6h) [C III] | ||
Parasites | Toxoplasma gondii | Pyrimethamine (orally, 100 mg load then 50 mg/d)b + sulfadiazine (orally, 50 mg/kg/d) [A I]c |
Alternative | ||
Pyrimethamine (orally, 100 mg load then 50 mg/d)b + clindamycin (600 mg q6h)d [B II]c or | ||
Trimethoprim/sulfamethoxazole (orally or intravenously, 10 mg/kg/d and 50 mg/kg/d) [B II]c | ||
Viruses | HHV-6 | Ganciclovir (5 mg/kg q12h) [B III] or foscarnet (60 mg/kg q8h) [B III]e |
EBV (virus encephalitis) | No antiviral treatment [C III] or ganciclovir (5 mg/kg q12h) [C III] | |
Acyclovir is not recommended [D III] | ||
HSV | Acyclovir (10 mg/kg q8h) [A I] | |
CMV | Ganciclovir (5 mg/kg q12h) or foscarnet (60 mg/kg q8h) as single agent [A III] or a combination of both [B III] | |
VZV | Acyclovir (10 mg/kg q8h) [A III] or ganciclovir (5 mg/kg q12h) [C III] | |
JC virus (PML) | Cidofovir is not recommended [D II] | |
Bacteria | Listeria monocytogenes | Ampicillin (2 g q4h) +/− aminoglycoside (for at least the first 7–10 days) [B III] or meropenem (2 g q8h) [C III] |
Pseudomonas spp. | Ceftazidime (2 g q8h) +/− aminoglycoside [B III] or | |
Meropenem (2 g q8h) +/− aminoglycoside [C III] | ||
Nocardia spp. | Imipenem (0.5 g q6h) + amikacin (5 mg/kg q8h) [B III]f or | |
Trimethoprim/sulfamethoxazole (5–10 mg/kg q12h)g + imipenem (0.5 g q6h) + amikacin (5 mg/kg q8h) [B III]f or | ||
Trimethoprim/sulfamethoxazole (5–10 mg/kg q12h)g + ceftriaxone (2 g/d) + amikacin (5 mg/kg q8h) [B III]f | ||
MSSA | Oxacillin (1.5–2 g q4h) [B III] or nafcillin (1.5–2 g q4h) [B III] or meropenem (2 g q8h) [B III] | |
MRSA | Vancomycin (0.5 g q6h or 1.0 g q12h) [B III] or linezolid (0.6 g q12h) [B III] | |
However, other measures can be applied in addition to anti-infective drug therapy in some situations. For example, a significant increase in survival has been achieved by combined neurosurgical and voriconazole treatment in patients with cerebral aspergillosis [55]. Indwelling CNS devices should always be removed in patients with suspected or proven CNS Candida infections [33]. A placebo-controlled trial demonstrated improved outcome in adult bacterial meningitis patients, who received concomitant glucocorticoid therapy, but this could not be confirmed by a more recently published meta-analysis [12, 68]. Concomitant therapy with voriconazole and glucocorticoids has also been successfully used in the treatment of CNS aspergillosis [24]. However, adjunctive glucocorticoid therapy cannot be generally recommended in hematology patients with CNS infections, since data on this approach are still limited and were mainly acquired in immunocompetent hosts.
13.2 CNS Infections Related to Specific Causative Agents
13.2.1 Fungi
Though rarely associated with CNS infections in non-immunocompromised hosts, fungi are among the most common causative agents in hematology patients, particularly after AlloSCT or in conjunction with neutropenia. The predominant fungal pathogens are Aspergillus spp., mainly A. fumigatus and less frequently other species such as A. nidulans, A. terreus, and A. flavus, whereas Candida spp. and Cryptococcus neoformans are only occasionally detected in these patients [20, 39, 55]. Patients with CNS aspergillosis typically present with persistent fever, altered mental status, and focal neurological symptoms. MRI may reveal areas consistent with infarction, ring-enhanced lesions due to abscess formation, or dural or vascular infiltration from adjacent regions (Fig. 13.2a) [18]. CSF fungal cultures are typically negative for Aspergillus spp. but recent observations indicate that CSF galactomanan or PCR assays might be useful tools to diagnose CNS aspergillosis in selected cases [2, 45, 58]. Definitive diagnosis of CNS aspergillosis frequently requires biopsy of lesions. Typical septate hyphae might be demonstrated after Grocott silver or H&E staining (Fig. 13.3).
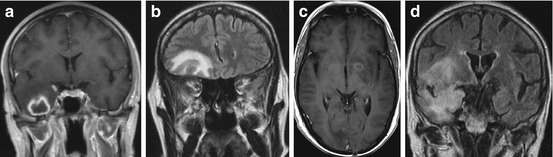
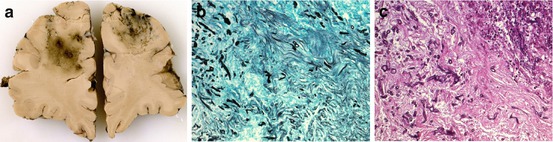

Fig. 13.2
Cranial MRI images illustrating typical abnormalities of different CNS infections in hematology patients. Shown are T1-weighted sequences after intravenous application of contrast medium (a, c) and dark fluid sequences (b, d). (a) CNS aspergillosis with a partially liquid lesion in the right temporal lobe surrounded by pronounced edema. (b) CNS mucormycosis with a large, frontobasal lesion and perifocal edema. (c) Neurotoxoplasmosis with multiple punctual and ring-configured hyperintense lesions in the cerebellum, basal ganglia, and cortical, subcortical, and subependymal regions. (d) Hyperintense laminar lesions with right frontotemporal preponderance in a patient with herpes encephalitis (The MRI images were kindly provided by W. Grassl, Clinic for Radiology and Nuclear Medicine, Charité Campus Benjamin Franklin, Berlin, Germany)

Fig. 13.3
(a) Gross photograph of a coronal brain section showing multiple ill-defined lesions in the frontal lobe in a patient with cerebral aspergillosis. (b, c) Photomicrographs (original magnification: ×200) show septate hyphae diffusely scattered throughout these lesions (Grocott silver stain (b) and H&E staining (c)) (The photographs were kindly provided by W. Stenzel, Department of Neuropathology, Charité Campus Mitte, Berlin, Germany)
Voriconazole should be preferred for CNS aspergillosis, since it has acceptable CSF penetration and a relatively favorable overall response rate of 35 % [55]. Liposomal amphotericin B might be a second treatment option (Table 13.2). Conventional amphotericin B deoxycholate should not be used anymore because of its unfavorable toxicity profile and inferior clinical efficacy [23]. The prognosis of CNS aspergillosis is still poor in adults with a mortality of about 70 %, but more favorable in children with a mortality of about 40 % (after 1990) [15, 55].
C. albicans and recently also other Candida spp. such as C. tropicalis or C. glabrata may cause meningitis presenting with subacute onset and with fever and headache [33]. However, Candida spp. may also cause cerebral microabscesses and occasionally macroabscesses presenting with focal neurological signs. Since CT and CSF analysis are rarely diagnostic for Candida microabscesses, they are often only diagnosed at autopsy [50]. In patients with Candida meningitis, CT might show hydrocephalus, and yeasts can be detected in the CSF in about 40 % by direct microscopy and in about 80 % by fungal cultures (Table 13.1) [50, 53]. Despite the lack of systematic data comparing MRI and CT in the diagnosis of Candida CNS infections, MRI is considered more sensitive and should thus be preferred. New techniques such as the PCR or detection of the Candida antigen mannan in the CSF might also be useful and are currently being further evaluated [3, 53]. Candida CNS infection might respond to voriconazole or liposomal amphotericin B, but only limited (mainly preclinical) data are available on their use in the treatment of Candida CNS infections [31, 51].
Cryptococcus neoformans typically causes meningitis. In hematology patients, it may present with acute or subacute onset and atypical symptoms, including fever, confusion, headache, or diplopia [39]. MRI may reveal signs of meningitis, dilated Virchow-Robin spaces, cyst-like structures, and granuloma of the choroid plexus [1, 62]. The diagnosis can be made in most cases by CSF fungal cultures, PCR analysis, and India ink smear microscopy (Table 13.1) [49]. Treatment of cryptococcal meningitis can comprise a combination of amphotericin B deoxycholate (preferentially as continuous infusion) and 5-fluorocytosine (Table 13.2) [4, 16, 41]. However, liposomal amphotericin B or voriconazole also seem to be useful treatment options since these two agents may have a better toxicity profile than amphotericin B deoxycholate [5, 9, 22, 25, 30, 41].
Mucormycosis is a rare opportunistic infection caused by Zygomycetes. The most frequent species include Absidia corymbifera, Rhizopus spp. and Apophysomyces elegans, but the spectrum shows geographic variations [6, 47]. It affects mainly the lungs and more rarely the soft tissue or the rhino-sinu-orbital region; the brain is involved in about 15 % of cases [47]. The rhinocerebral type typically presents with facial pain and swelling. Though this disease is often suspected initially from clinical appearance and imaging results (Fig. 13.2b), the diagnosis should always be confirmed by biopsy. Liposomal amphotericin B now seems to be the most active anti-infective agent for invasive mucormycosis (Table 13.2) [10, 40, 47, 56, 61]. Preliminary data further suggest that a combination of (liposomal) amphotericin B and caspofungin might be useful to treat invasive [10, 27, 44].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree