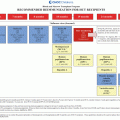
Disease
Treatment
Potential CNS late effects
Craniopharyngioma
Surgical resection/biopsy, cyst fenestration, radiation therapy
Panhypopituitarism, obesity, hypogonadism, lower physical health and psychosocial difficulties
Optic Pathway Glioma
Chemotherapy (Carboplatin, Vincristine, CCNU, 6-thioguanine, procarbazine) for younger patients, local radiation (XRT) for older patients or recurrent tumors, surgical resection in selected patients
Visual impairment, growth hormone deficiency, hypothyroidism, endocrinopathies, neuropathy, neurocognitive deficit
Medulloblastoma
Surgical resection, Craniospinal XRT with boost to the posterior fossa, Chemotherapy (Cisplatin, CCNU, Cyclophosphamide, Vincristine, carboplatin)
Ataxia, cranial nerve palsy, diplopia, poor balance, hearing loss, growth failure, impaired spinal growth, neurocognitive deficit, shunt failure, second malignancy [47]
Supratentorial PNET
Surgical resection/biopsy, craniospinal XRT with boost to the tumor bed, chemotherapy (Cisplatin, Cyclophosphamide, vincristine, Carboplatin)
Seizure, motor deficit, poor hand-eye coordination, lower IQ, neurocognitive deficit, poor memory, attention deficit, emotional difficulties, growth failure, endocrinopathies, hearing loss
Ependymoma
Surgical Resection, radiation to the tumor bed for non-metastatic disease, additional multiagent chemotherapy to patients with incompletely resected tumors
Cranial nerve deficits, abnormal gait, fine motor function deficit, memory loss, dysphagia, truncal ataxia, neurocognitive deficit
Low grade astrocytoma or other glial tumor
Surgical resection. Radiation to the residual or recurrent tumor, chemotherapy for younger patients
Blindness, hearing loss, obesity, endocrinopathies (diencephalic location), lower IQ [48]
High grade astrocytoma or other glial tumor
Surgical resection. Radiation to the tumor bed, chemotherapy (Temozolomide, CCNU, Bevacizumab, irinotecan)
Neurocognitive deficit, seizure, headache, regional based late effects
Choroid Plexus Tumors (CPT)
Surgical resection, Etoposide, carboplatin and other chemo for high grade CPT
Hydrocephalus/shunt dysfunction, motor dysfunction, psychomotor retardation
CNS Germ Cell Tumors (GCT), (supraseller or pineal)
Third ventriculostomy, biopsy/resection Radiation to the ventricular system or neuraxis± platinum based chemotherapy
Neurocognitive deficit, diabetes insipidus, hypopituitarism (supraseller GCT), neurocognitive dysfunction
Infant embryonic tumors
May receive less or no radiation therapy but more aggressive chemotherapy with autologous stem cell support
Lower visual-motor integration. Lower IQ, hearing loss, seizure, second malignancies
Acute Lymphoblastic Leukemia
Intrathecal chemo for all patients, cranial XRT for selected patients (CNS leukemia or very high risk ALL or patients treated before 1990), multiagent chemotherapy including methotrexate, vincristine, steroid, anthracycline, mercaptopurine, asparaginase
Headache, auditory-vestibular-visual sensory deficit, coordination and motor sensory disorders, seizures, brain tumor and other second neoplasms, neurocognitive deficit
CNS-directed therapy may alter cognitive, emotional and physical performance [49]. Chronic fatigue and pain are common complaints among survivors [50, 51]. A summary of these effects are presented in Table 5.2, and are discussed below.
Table 5.2
Clinical presentations of CNS late effects
Type of late effects | Clinical presentation | Risk factors |
|---|---|---|
General | Low physical performance Lower educational achievement Not living independently | Cranial radiation, neurological deficits |
Neurological | Leukoencephalopathy | Radiation, high dose methotrexate |
Coordination and motor control disorders | Radiation >50 Gy to the frontal brain | |
Seizure disorder | Radiation >30 Gy to cerebral cortex regions | |
Stroke | Cranial radiation >30 Gy | |
Visual impairment | Optic pathway tumors | |
Hearing impairment Tinnitus | Radiation >50 Gy to the posterior fossa, platinum chemotherapy | |
Neurocognitive | Poor attention Slow processing speed Lower visual-perceptual skills Lower executive function Poor memory Lower IQ and academic achievement | Young age at diagnosis Cortical tumors >35 Gy radiation to frontal lobe >24 Gy cranial radiation Hydrocephalus, VP shunt placement Neurosensory deficits |
Endocrinological | Growth failure | >18 Gy radiation to H-P axis, age <4 years at diagnosis |
Gonadal dysfunction Central hypothyroidism Central adrenal insufficiency Diabetes insipidus | Cranial radiation ≥40 Gy | |
Obesity | Female sex, young age at diagnosis, >20 Gy radiation to H-P axis | |
Early menarche | <4 years old at diagnosis | |
Late menarche | >50 Gy radiation to H-P axis | |
Second malignancies | Gliomas Meningiomas | ≤5 years old received CNS radiation, ≥50 Gy CNS radiation, NF1 |
Psychological | Depression Anxiety Somatic distress Daytime sleepiness Social withdrawal Poor self-concept | Major medical conditions Female sex Lower socioeconomic status Lower education achievement |
Social outcomes | Educational difficulties Lack of friends Unemployment Not married | Cranial radiation therapy Young age at the diagnosis Female sex |
In the CCSS study, 82 % of the 1,877 adult survivors of childhood CNS malignancies reported at least one chronic medical condition, with 38 % reporting a serious life-threatening condition. Endocrine complications and neurologic complications are most commonly reported [52]. Among all childhood cancer survivors, survivors of CNS malignancies have the highest risk for grade 3 or 4 chronic health conditions using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) [53]. They also reported significantly higher rates of global distress, depression, and somatic distress than their siblings [3].
Among ALL survivors studied by the CCSS, one or more chronic medical conditions were reported by 50 % of survivors, compared with 37.8 % of siblings. Severe chronic health conditions were more commonly reported in survivors who received radiation therapy or experienced relapses [54].
5.4.1 Endocrinological Late Effects
Brain tumor survivors with tumor in the hypothalamic-pituitary axis (H-P axis) and those who received more than 24 Gy cranial radiation are at high risk for neuroendocrine dysfunction. The endocrinological late effects include growth failure, gonadal dysfunction, central hypothyroidism, central adrenal insufficiency, obesity and diabetes insipidus. Cranial radiation doses as low as 18 Gy can affect the growth hormone axis [55]. Panhypopituitarism, a condition in which there is inadequate or absent production of the anterior pituitary hormones, usually develops in patients who have received greater than 40 Gy of cranial radiation [56]. Gonadal dysfunction includes precocious puberty, delayed puberty and/or infertility. The CCSS reported decreased fertility among female childhood cancer survivors who received 22–27 Gy H-P axis irradiation [57]. Hyperprolactinemia can also occur in patients who received radiation to the hypothalamic/pituitary area, producing symptoms of decreased libido, galactorrhea, and amenorrhea [58].
The CCSS cohort (n = 1,607) showed that childhood brain tumor survivors had a significantly increased risk of late-onset hypothyroidism with relative risk ratio (RR) of 14.3, growth hormone deficiency (RR = 277.8), the need for medications to induce puberty (RR = 277.8) and osteoporosis (RR = 24.7) [56]. Female survivors were more likely to have onset of menarche before age ten compared to their siblings (11.9 % vs. 1.0 %). Age ≤4 years at diagnosis was associated with an increased risk of early menarche. Additionally, survivors of CNS tumors were more likely than siblings to have onset of menarche after age 16 (10.6 % vs. 1.9 %). Doses of RT to the H-P axis >50 Gy and spinal RT conferred an increased risk of late menarche, as did older age (>10 years) at the time of diagnosis [59]. As a result, H-P axis radiation and chemotherapy with alkylating agents reduce the likelihood of pregnancy among female survivors [60].
The strongest risk factors for adult short stature were 4 years of age or younger at diagnosis and radiation treatment involving the H-P axis [61]. The risk of metabolic syndrome and/or overweight/obesity increases for those less than 4 years old at time of treatment, female sex, hypothalamic radiation dose >20 Gy and inability to exercise due to physical limitations.
5.4.2 Neurological Late Effects
Leukoencephalopathy (LE) can develop after exposures to mid-to-high dose methotrexate. This is best detected on the T-2 weighted and FLAIR images of MRIs with the prevalence ranging from 21 % to 76 %. Increasing exposure, which corresponded to more courses and higher doses of IV MTX, was a risk factor for LE. Some of the LE changes were transient, as evidenced by a significant reduction in the prevalence of LE approximately 1.5 years after the completion of IV MTX therapy [62, 63].
Coordination and motor control disorders were reported in 49 % and 26 % of survivors, respectively. Children receiving at least 50 Gy to the frontal brain region had a modestly elevated risk for motor problems [64].
Seizure disorders were reported in 25 % of survivors of brain tumor, including 6.5 % who had a late first occurrence. Radiation dose of 30 Gy or more to any cortical segment of the brain, with the exception of the posterior fossa, was associated with a two-fold elevated risk for a late seizure disorder [52, 64]. Among acute leukemia survivors in the CCSS cohort, 7 % had seizures, the majority of which was late onset [54].
Stroke has been observed in survivors of childhood leukemia and brain tumors from the CCSS cohort, particularly those with brain tumors treated with greater than 30 Gy of cranial radiation are at an increased risk of stroke [65]. The relative risk of stroke for survivors was 6.4 for leukemia and 29 for brain tumor survivors compared with the sibling comparison group. In leukemia survivors, the risk of late-occurring stroke compared to siblings was increased for survivors treated with cranial radiation (RR, 5.9) and without cranial radiation (RR, 4.0). The cumulative incidence of stroke was 0.73 % at 25 years after treatment for leukemia survivors and 5.58 % for brain tumor survivors [65]. Severe recurrent headache may be a predictor for subsequent stroke or TIA [66].
Moyamoya syndrome is a potentially serious complication of cranial irradiation in children, particularly for those patients with tumors in close proximity to the circle of Willis, such as optic pathway glioma. It is a progressive vascular occlusive disease with particular involvement of the circle of Willis, manifesting as stroke, or recurrent transient ischemic attacks (TIA). Patients with NF1 also have increased risk of developing moyamoya syndrome (HR = 3.01) [5].
Neurosensory deficit has been noted in 17 % of CCSS patients. Eye problems, including ocular nerve palsy, double vision, gaze paresis, nystagmus, papilledema, optic atrophy, visual loss and cataracts are common in childhood brain tumor survivors. The CCSS study showed brain tumor survivors were at substantial elevated risk for late-onset legal blindness in one or both eyes (RR, 14.8). Hearing impairment was reported by 12 % of patients. Radiation exposure of greater than 50 Gy to the posterior fossa was associated with a higher likelihood of developing any hearing impairment [64]. Tinnitus was common both early and late in the illness. It is likely that wider use of cisplatin and high dose carboplatin after 1986 will increase the rate of hearing impairment [67]. Auditory-vestibular-visual sensory deficits were reported in 15.1 % of ALL survivors in the CCSS study. Serious headache were most common in ALL survivors with a cumulative incidence of 25.8 % at 20 years [54].
Chronic progressive radiation myelopathy can occur months to years following spinal radiation manifested with bilateral leg paresthesias, weakness, and painful, electric-like shock sensation elicited on neck flexion (Lhermitte’s sign) [68].
5.4.3 Neurocognitive Late Effects
It is well known that survivors of childhood ALL and CNS malignancies are at greatest risk for neurocognitive impairment, particularly if they have received cranial radiation. While this manuscript will address this particular issue in separate chapters, a brief review for the reader is hereby offered. The neurocognitive sequelae are most apparent in attention, processing speed, visual perceptual skills, executive function (planning and organization) and memory. Deficits in these areas result in declines in IQ, reading comprehension, spelling, mathematics, skill acquisition and academic achievement.
Cognitive growth is also reduced in survivors, so the cognitive gap between the survivors and the general population increases with time [69]. Decreased white matter volume (WMV) was shown in the survivors of childhood ALL and malignant brain tumors which were associated directly with lower scores in intelligence, attention, and academic performance. Increased CNS treatment intensity, younger age at treatment and greater time since treatment were significantly associated with lower WMV [70]. Reported neurocognitive impairment adversely affected important adulthood outcomes, including education, independent living, employment, income and marital status [71].
Factors within the population of pediatric CNS malignancy survivors that impacted neurocognitive outcome include tumor type and site [72], age at diagnosis, and dose and volume of radiation therapy [73, 74]. Cortical tumors have been reported to result in more cognitive late effects than 3rd or 4th ventricle tumors [71, 72]. Survivors who received high-dose cranial radiation to frontal areas of their brain (i.e. >35 Gy) reported significantly more problems with attention, processing speed, memory and emotional regulation [75]. However, pediatric posterior fossa tumors have also been associated with neurocognitive sequelae including deficits in attention, planning, sequencing, executive functioning, memory, processing speed, visual-spatial organization, and modulation of affect and behavior [76–78].
Although children with pilocytic astrocytoma (PA) generally carry a better prognosis and many were treated with surgery only, the CCSS study showed that survivors of astrocytoma have high rates of impairment in attention, processing speed and memory. Impairment increases with cranial radiation exposure [3]. Aarsen et al conducted a prospective neurocognitive study of 67 children treated for PA and found that cognitive impairments are common. All children with PA had problems with sustained attention and speed. In the infratentorial group, there also were deficits in verbal intelligence, visual-spatial memory, executive functioning, and naming. The supratentorial hemispheric tumor group had additional problems with selective attention and executive functioning. More specifically, the dorsal supratentorial midline tumor group displayed problems with language and verbal memory. Predictors for lower cognitive functioning were hydrocephalus, radiotherapy, residual tumor size, and age. Predictors for better functioning were chemotherapy or treatment of hydrocephalus. Almost 60 % of children had problems with academic achievement, for which risk factors were relapse and younger age at diagnosis [79].
Survivors of medulloblastoma demonstrate a decline in IQ values because of an inability to acquire new skills and information at a rate comparable to their healthy same-age peers, as opposed to a loss of previously acquired information and skills [80]. Only 30 % of young adults who were survivors of medulloblastoma were able to drive, live independently, or find a job [81]. Over 40 % of medulloblastoma survivors had impairment in attention and processing speed regardless of RT exposure [3, 71]. Younger age at diagnosis, high-risk disease and higher baseline scores were significantly associated with poorer outcomes in processing speed, working memory and attention over time [82]. Severe hearing impairment and posterior fossa syndrome were associated with poor neurocognitive outcomes particularly with reading ability and language skills [83].
Survivors of ALL have been found to display deficits in a variety of executive functions, including working memory. These deficits have been hypothesized to develop as a result of chemotherapeutic and/or corticosteroid agents that are administered during prophylactic treatment [84, 85]. Survivors who received cranial radiation have more impairment in memory and motor functions than non-radiated survivors [86, 87]. Female survivors diagnosed at young age performed worse in the scholastic testing [14]. Impairment in executive function skills increased with time since diagnosis, and appear to be related to functional outcomes as adults, including college graduation and full-time employment [88].
5.4.4 Psychological Late Effects
Zebrak et al. evaluated and compared psychological outcomes in long-term survivors of pediatric brain cancer and siblings of childhood cancer survivors [89]. Survivors of childhood brain cancer in the CCSS cohort appear to report significantly higher global distress and depression scores than their siblings. There is a correlation between health status and psychological functioning [89]. Those survivors with a history of a major medical condition reported more symptoms of depression, anxiety and somatic distress. As in the general population, higher levels of distress among survivors were associated with female sex, low household income, lower educational attainment, being unmarried, not being employed in the past 12 months, and poor physical health status [89]. CCSS study also showed brain tumor survivors as a particularly vulnerable group, with more psychological distress, fatigue, cognitive problems and diminished life satisfaction than other survivors. Survivors scored lower than population norms for most aspects of Health Related Quality of Life (HRQOL) measures. An increased risk of hospitalization for psychiatric disorders was observed among survivors of brain tumor [90] as well as suicide ideation [91, 92].
CNS radiation is linked to impairment in physical health, more functional impairment and more activity limitations and increase sleep disruption and fatigue. Leukemia survivors experienced increased rates of depression, anxiety and social-skills deficits compared with sibling controls during the adolescent period [75].
5.4.5 Social Outcomes
Measurement of social outcomes including education, employment, relationship and independent living closely relates to the quality of life of survivors. Diagnosis and treatment of cancer can have a great impact in social development. CNS impairment can further compromise social outcomes.
Educational Problems. Among the CCSS cohort, survivors of brain tumors and leukemia were most likely to have educational problems and no close friends [93]. Eighteen percent of young adult survivors of brain tumors had not completed high school. Seventy percent of brain tumor survivors diagnosed before the age of 6 years required special education services in school [3]. Ness et al. conducted a study of 156 adult survivors of childhood brain tumors and compared them with population-based comparison group. Physical performance (muscle strength and fitness values) was lower among survivors and was associated with not living independently and not attending college [94].
Employment and Marriage. CNS tumor survivors had the highest risk of unemployment (odds ratio = 9.9) [95]. Risk for unemployment increased with chronic medical conditions after cancer therapy, young age (<4 years) at diagnosis, cranial radiation therapy of ≥30 Gy, and female sex [93, 95]. There was a dose-dependent association between RT to the frontal and/or temporal lobes and lower rate of employment, less household income and less likely to get married [52]. Seventy-eight percent of brain tumor survivors had never married compared to 62 % of the whole CCSS cohort [96]. Many brain tumor survivors are unable to live independently.
Children with ALL who did not receive radiation therapy and who have attained 10 or more years of event-free survival can expect a normal long-term survival. However, irradiated leukemia survivors have a slight excess in mortality, and an increased unemployment rate. Women in the irradiated group were less likely to be married [93, 97]. ALL survivors receiving cranial radiotherapy of ≥24 Gy or age less than 6 years old at diagnosis are more likely to require special education program [98].
5.4.6 Second Malignancies Affecting Central Nervous System
Within the CCSS cohort of 14,361 5-year survivors, subsequent CNS primary neoplasms were identified in 116 individuals. Radiation therapy was associated with a dose-dependent increased risk for subsequent gliomas (odds ratio = 6.78, 95 % CI 1.54–29.7) and meningiomas (odd ratio 9.94, 95 % CI 2.17–45.6). The relative risk per Gy for glioma was highest among children treated with radiation at age 5 years or younger. The risk of glioma was increased within 5–10 years after radiation, but declined to nearly background levels after 15–20 years. In contrast, the incidence of meningioma increased steadily from 5 to 10 years after radiation and showed no evidence of plateau [99]. The median age at diagnosis of glioma was 15 and 25.5 years for meningioma [100]. Among survivors of CNS malignancies, 76 subsequent malignant neoplasms were reported among 1,877 survivors. The most common second malignancies were CNS tumors (20 observed), followed by thyroid cancer (12 observed) and soft tissue sarcomas (eight observed) [3]. Survivors of CNS malignancies who received CNS-directed radiation >50 Gy had a cumulative incidence of a subsequent CNS neoplasm of 7.1 % at 25 years compared with 5.2 % for those receiving <50 Gy and, 1.0 % for those who received no CNS radiation [16, 52, 100]. Death from the second malignant neoplasms accounts for 10 % of late death of brain tumor survivors [101].
Patients with neurofibromatosis are at higher risk for developing secondary malignancy following radiation. The relative risk of second nervous system tumor after radiotherapy was 3.04 (95 % CI, 1.29–7.15). There is a significantly increased risk of second nervous system tumors in NF1 patients who received radiotherapy for optic pathway glioma, especially when treated during childhood [4].
In the report by Packer et al from a Phase 3 study of children with average risk medulloblastoma treated with craniospinal radiation therapy followed by adjuvant chemotherapy, second malignancies including myelodysplastic syndrome (MDS), pilocytic astrocytoma, T-ALL, malignant glioma (cerebellar), basal cell carcinoma, and glioblastoma were reported to develop 38–76 months from the study enrollment [47].
Radiation associated CNS neoplasms have been reported as the most common second malignancy among survivors of childhood leukemia [102]. CCSS study reported 199 second neoplasms (SN) developed in 4,151 ALL survivors. Eighty-one percent of SNs developed in survivors who received radiation therapy, with 53 % of SNs occurring in the CNS including meningioma (66), astrocytoma (22), medulloblastoma (3) and others (15) [54].
5.4.7 Late Mortality
Among childhood cancer survivors treated from 1970 through 1986 followed by CCSS, the CNS tumor survivors had the worst overall survival with a cumulative mortality rate of 17.1 % at 20 years. The major cause of death (67 %) among the 5-year survivors was recurrence or progression of the original CNS malignancy. Survivors of medulloblastoma or primitive neuroectodermal tumor have a 17-fold higher risk of death than that of the general population. Recurrence or progression was the leading cause of death until 30 years after diagnosis [52]. The risk for death from disease recurrence was greatest in the time period of 5–9 years after initial diagnosis. This pattern of late recurrence suggests a need for continued surveillance of disease well beyond the first 5 years from diagnosis.
5.5 Surveillance of Late Effects
Improvements in treatment of brain tumor and leukemia have increased the rate of survival. Recognition of many CNS late effects in survivors of brain tumors and leukemia is important for early intervention and treatment. Survivors should receive risk-based surveillance based on prior treatment and have a plan for lifetime follow-up for potential late effects [60]. The medical team should ensure that survivors have access to multidisciplinary specialists including an oncologist, neurologist, neurosurgeon, endocrinologist, psychologist, speech and occupation and physical therapist, ophthalmologist, dentist, and audiologist as needed.
Table 5.3 summarizes the surveillance for potential CNS late effects based on risk factors. Obtaining the survivor’s medical history and treatment summary can identify risk factors and direct the modality of surveillance. It is important to recognize that the pattern and incidence of late effects may change over time, with the evolution of treatment for leukemia and CNS tumors.
Table 5.3
Surveillance for potential late effects of the central nervous system
Potential late effects | Risk factors | Evaluation | Further testing and intervention |
|---|---|---|---|
Brain Tumors (primary or secondary) | Cranial radiation particularly in patients with neurofibromatosis (NF) or younger age at treatment | Yearly neurological exam Brain MRI for symptomatic patient. MRI every other year for patients with NF | Neurosurgical and Neuro-Oncology consultation |
Structural Damage of CNS | |||
Hydrocephalus Shunt malfunction | Primary CNS tumors | Evaluation by neurosurgeon Brain CT as indicated | Patient/family education regarding potential symptoms of shunt malfunction |
Movement Disorders, Ataxia, Paralysis, peripheral neuropathy | Primary CNS tumors Brain stem and cerebellar tumors Vinca Alkaloid | Evaluation by neurologist, rehabilitation specialists as indicated | Physical and/or occupational therapy |
Leukoencephalopathy ( spasticity, ataxia, dysarthria, dysphagia, hemiparesis, seizures | Cranial radiation dose ≥24 Gy Young age at treatment Methotrexate (IT. IO. High-dose IV) High dose Cytarabine | Yearly review of neurological symptoms and neurological exam MRI brain with diffusion-tensor imaging to evaluate white matter MR angiography Brain CT looking for calcifications | Physical, occupational and speech therapy Neurology consultation |
Cerebrovascular complications (stroke, Moyamoya, occlusive vasculopathy) | Parasellar tumor and radiation dose ≥50 Gy Sickle cell disease Neurofibromatosis | History of hemiparesis, hemiplegia, weakness, aphasia Yearly neurological exam Brain MRI with diffusion-weighted imaging and MR angiography as clinically indicated | Physical, occupational and speech therapy as clinically indicated Revascularization procedure for Moyamoya may be helpful |
Seizure Disorder | Primary CNS tumors Methotrexate (IV, IT, IO) | Neurologist evaluation every 6 months for patients with seizure disorder | anticonvulsants |
Neurogenic bladder/bowel | Spinal cord tumor/surgery Radiation dose ≥ 45 Gy to lumbar/sacral spine and/or cauda equina | Evaluate urinary symptoms (urgency, incontinence, retention, dysuria) Evaluate bowel symptoms (chronic constipation, fecal soiling) | Urology consultation Bowel regimen (fiber, laxatives, enema) as indicated GI consultation as indicated |
Neurosensory deficits | |||
Visual Deficit (ocular nerve palsy, gaze paresis, nystagmus, papilledema, optic atrophy, cataract, low vision, visual field defect) | Higher radiation dose Optic pathway tumor Suprasellar tumor | Yearly eye exam (visual acuity, optic nerve disc, cataract, ocular nerve, eye irritation) | Ophthalmology evaluation and intervention |
Hearing impairment | Higher radiation dose, chronic otitis, ototoxic agents (Cisplatin, Carboplatin, Aminoglycosides) | Yearly otoscopic exam Complete audiological evaluation yearly after completion of therapy for 5 years, then every 5 years. Yearly if hearing loss is detected | Otolaryngology and Audiology consultation for hearing aid Preferential classroom seating, FM amplification system, other educational assistance |
Hypopituitarism | |||
Central hypothyroidism | Cranial Radiation dose ≥40 Gy | Yearly height, weight, hair, skin and thyroid exam TSH, free T4 yearly | Endocrine consultation for thyroid hormone replacement |
Central adrenal insufficiency | Cranial Radiation dose ≥40 Gy | History- failure to thrive, anorexia, dehydration, hypoglycemia, lethargy, hypotension Yearly 8 am serum cortisol level | Endocrine consultation Corticosteroid replacement and stress dosing |
Growth failure | Cranial Radiation dose ≥18 Gy, younger age at treatment, pretransplant cranial radiation or total body irradiation (TBI) | Assessment of growth and Tanner staging every 6 months until growth and sexual maturation completed
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |