Funding
This work was supported by National Institutes of Health (NIH) grants P01 AG062413 (J.N.F.), R21 AG065868 (J.N.F), K01 AR070241 (J.N.F.), as well as a High-Risk Pilot Award (J.N.F.) and Career Development Award (J.N.F.) from the Mayo Clinic Robert and Arlene Kogod Center on Aging, and the Richard F. Emslander Career Development Award in Endocrinology (J.N.F.).
Conflict of interest
Japneet Kaur and Joshua Farr have nothing to disclose.
Introduction
Aging is associated with universal cellular damage and the simultaneous decline of several crucial biological processes. Lopez-Otin et al. grouped these fundamental mechanisms or “ hallmarks of aging ” into nine categories as follows: telomere attrition, genomic instability, loss of proteostasis, epigenetic alterations, mitochondrial dysfunction, deregulated nutrient signaling, stem cell exhaustion, altered intercellular communication, and cellular senescence. Each of these hallmarks of aging meets the following criteria: (i) it manifests during normal chronological aging; (ii) its experimental induction accelerates aging; and (iii) its experimental amelioration slows or delays the natural aging process and hence extends healthspan (i.e., the period of life free of chronic disease).
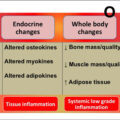
There is now mounting evidence that each of these hallmarks of aging apply to essentially every tissue and system throughout the body, including bone and skeletal muscle (as previously reviewed ). Thus, there is direct experimental data in several preclinical models and some evidence in humans for each of the nine fundamental aging mechanisms to have causal roles in mediating multiple diseases and co-morbidities of aging, including osteoporosis and sarcopenia. The extent to which individual aging hallmarks drive aging is important because these mechanisms are linked and overlap, and thus each contribute to the overall aging process . Indeed, based on their interconnections and overlap, interventions that therapeutically target one hallmark should in theory prevent or delay age-related co-morbidities across tissues and systems. Therefore, interfering with one hallmark has the potential to simultaneously ameliorate a majority of age-associated chronic diseases as a group. This approach is fundamentally different that the current paradigm of one drug to treat each disease of aging, which ultimately leads to polypharmacy and potential adverse drug interactions in the older population. One such hallmark of aging that fits the above criteria and appears to be particularly amendable to therapeutic targeting to prevent multiple age-related co-morbidities, at least in mice, is cellular senescence. Here we review the evidence for reducing the cellular senescence burden to alleviate musculoskeletal aging.
Cellular senescence
Cellular senescence is a cell-intrinsic phenomenon that has been observed both in vitro and in vivo. The senescence pathways are triggered by various forms of cellular stress or metabolic insults that cause the accumulation of cellular damage. Specific examples of intrinsic and extrinsic stressors that converge to cause a cell to enter the senescence program include DNA damage, telomere shortening, oxidative and proteotoxic stress, activation of oncoproteins such as Ras and its sequelae, as well as prolonged shifts in nutrient homeostasis in the forms of both deprivation and excess. Over time, these cumulative insults can eventually cause a cell to enter an essentially irreversible proliferative arrest, making the cell resistant to apoptosis yet metabolically active and immune to mitogenic stimuli. Senescent cells undergo dramatic changes in gene expression, chromatin organization, and morphological changes resulting in cell enlargement and flattening . Although not always the case, senescent cells frequently produce a pro-inflammatory secretome that contributes to the spread of senescence and is responsible for driving damage across tissues .
First described by Dr. Leonard Hayflick in the early 1960s , cellular senescence was demonstrated in a series of elegant cell culture experiments establishing that normal embryonic human fibroblasts grown in culture cease to divide after numerous cell divisions, even under favorable conditions. These experiments revealed that cells initially start dividing gradually (Phase I), which is followed by a period of profuse cell growth (Phase II). After phase II cells gradually lose their ability to divide and enter phase III, termed “cellular senescence” . The number of cell divisions or population doublings that a cell undergoes before reaching the senescence phase (which differs among cell types and species) is referred to as the Hayflick Limit . For example, human fibroblasts undergo ~ 50 population doublings (or cell divisions propagated in a 1:2 ratio) before they enter the senescence phase . It was later established that the reason there is an inherent limit to cell division is because with each mitosis, telemetric DNA shortens, which after many population doublings makes cell division impossible .
Telomeres are functional chromosomal ends that protect the genome. They are rich in 5′-TTAGGG-3′ nucleotide sequence repeats; however, with each ensuing DNA replication the terminal segment of the telomere in the lagging strand is lost, leading to telomere attrition over time. On average, human telomeres shorten at a rate of 24.8 to 27.7 base pairs per year with the rate of shortening directly related to the biological age of the individual and the risk for age-related diseases . This loss of sequence from the ends of the chromosomes can be reversed by telomerase, an enzyme responsible for synthesizing chromosomal ends by elongating telomeres. Nonetheless, most human somatic cells have minimal or no telomerase activity and thus their telomeres shorten with each replication until their length reaches a critical threshold which triggers cellular senescence—termed “replicative senescence.” Telomere shortening is thus considered a “ mitotic clock ” that can be used to estimate the history of replication for a given cell. It has been hypothesized that the amount of telomere shortening occurring with each division remains constant in a particular cell population; however, the rate of shortening can increase in response to various types of cellular stress resulting in premature telomere shortening and senescence of the cell at an earlier age . Poor lifestyle habits and other risk factors such as cigarette smoking, obesity, anxiety, and stress have been linked to accelerating the pace of telomere shortening in human cells .
The shortening of telomeres leads to their “uncapping”—i.e., loss of the terminal t-loop configuration, which can activate a DNA damage response (DDR) . This genomic damage caused by telomere shortening or due to other insults such as ionizing radiation as well as chemotherapeutic agents mimics DNA double-stranded breaks (DSBs) that trigger the DDR through the activity of sensory kinases (ATM/ATR, DNA-PK), resulting in the formation of DNA damage foci, and phosphorylated histone H2A.X (γH2A.X). This in turn can trigger the tumor suppressor protein, p53, to activate its downstream transcriptional target, p21 Cip1 (a cyclin-dependent kinase inhibitor [CDKi], also known as Cdkn1a ), thus halting the cell-cycle. If, however, the nature of the damage is severe and irreparable, p53/p21 Cip1 signaling will persist and thereby activate other signaling pathways such as p38 MAPK and protein kinase C, upregulating the expression of p16 Ink4a (a CDKi encoded by the Ink4a/Arf locus, also known as Cdkn2a ) which activates Rb, another tumor suppressor protein, thereby causing a more persistent and pronounced cell-cycle arrest . Cellular senescence triggered via the DDR can also occur through direct activation of the p16 Ink4a /Rb pathway, although in most cases p16 Ink4a is activated secondary to the p53/p21 Cip1 pathway . Some stressors, such as elevated reactive oxygen species (ROS) can induce senescence through direct activation of p16 Ink4a /Rb resulting in a persistent cell-cycle arrest . Thus, cellular senescence is activated and maintained by chronic signaling of either the p53/p21 Cip1 or p16 Ink4a /Rb tumor-suppressor pathways, or by both .
Senescence-associated secretory phenotype (SASP)
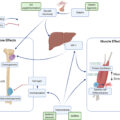
Persistent DDR signaling and the enhanced metabolic activity of senescent cells can result in revved-up intracellular machinery that permits the secretion of a complex S enescence- A ssociated S ecretory P henotype (termed, the “ SASP ”) typically comprised of cytokines, chemokines, matrix-degrading remodeling proteins (e.g., MMPs), and growth factors . Important SASP regulators in senescent cells can include IL-1α, IL-6, NFκB, C/EBPβ, or GATA4 . Although the exact composition of the SASP is dependent on the cell type and the type of the senescence inducing stimuli, SASP components are generally pro-inflammatory in nature, including factors such as interleukins, various monocyte chemoattractant proteins and macrophage inflammatory proteins, as well as granulocyte/macrophage colony-stimulating factors, and MMPs . Elevated SASP factors have been shown to mediate the spread of senescence to neighboring, nonsenescent cells (at least in vitro) via the senescence-induced bystander effect, resulting in a greater accumulation of senescent cells and further amplification of the SASP . Likewise, through this spreading mechanism an elevated in vivo senescent cell and SASP burden may, at least in part, propel the chronic, sterile low-grade inflammation associated with aging (termed “ inflammaging ”) that has been hypothesized to contribute substantially to morbidity and mortality . Therefore, senescent cells represent promising therapeutic targets to prevent or delay age-related diseases. Indeed, mounting evidence, predominantly from mice and more recently from studies in humans, has shown that senescent cells and their accompanying SASP increase with aging and in the context of several age-associated co-morbidities such as metabolic dysfunction, hepatic steatosis, neurodegenerative diseases, renal disease, pulmonary fibrosis, cardiovascular disease, osteoarthritis, frailty, sarcopenia, and osteoporosis .
Senescent cells have the ability to protect themselves from their own pro-apoptotic SASP via a pro-survival mechanism that involves the upregulation of multiple S enescent C ell A nti-apoptotic P athways , termed “ SCAPs ” . Among those SCAPs that have been discovered to date, include the B-cell lymphoma (BCL) family inhibitors (BCL-2, BCL-xL, BCL-W), PI3K/AKT pathways, p53/p21 Cip1 /serpine pathways, HSP-90 pathway, serpine (PAI-1, PAI-2), HIF-1α, ephrins, and dependence receptors/tyrosine kinases . Originally coined as the “ Achilles’ Heel ” of the senescent cells, SCAPs are the targets of drugs referred to in the field as “ senolytics ” that selectively kill senescent cells by inducing their apoptosis without having detrimental effects on nonsenescent cells . Among the first-generation senolytic compounds identified were Dasatinib (D; an FDA-approved tyrosine kinase inhibitor) and Quercetin (Q; a flavonoid present in many fruits and vegetables) that, when administered intermittently have both in vitro and in vivo senolytic activity .
Given that senescent cells are present at the time and location of aging and diseases in both animals and humans alike, there has been heightened recent interest in the therapeutic potential of senolytics to delay aging and extend healthspan . In addition to using senolytics, in order to examine the mechanisms and consequences of senescent cell removal in preclinical animal models of aging and various diseases, several groups have developed transgenic mouse models that harbor “suicide” transgenes driven by senescence gene promoters. For example, the “ INK-ATTAC ” (i.e., p16 Ink4a -linked apoptosis through targeted activation of caspase ) mouse model permits inducible removal of cells positive for p16 Ink4a (a marker expressed at high levels in many types of senescent cells) by activation of an FKBP-caspase 8 fusion protein that dimerizes upon administration of a synthetic drug, AP20187, which in turn activates an apoptosis cascade within p16 Ink4a -positive senescent cells to permit their inducible killing or “clearance” . In addition, p16 Ink4a trimodality reporter (p16-3MR) transgenic mice have been developed to harbor a different senescent cell killing system which utilizes ganciclovir as an activator molecule that triggers a mitochondrial DNA damage cascade in an inducible manner to specifically eliminate p16 Ink4a -positive senescent cells . Several studies, some of which are described below, have used either genetic or senolytic approaches (or the combination of both strategies) in old mice and models of accelerated age-associated diseases to study the biological functions of senescent cells in vivo as well as the consequences of their selective removal .
Biomarkers of cellular senescence
Although a single universal marker to unequivocally identify a senescent cell has yet to be discovered, p16 Ink4a appears to be expressed at higher levels in many types of senescent cells in both human and rodent tissues and thus far has been regarded by many as a critical senescent cell-specific marker . Nevertheless, because not all senescent cells express high levels of p16 Ink4a , the field has recommended the application of multiple criteria to identify and characterize senescent cells both in vitro and in vivo . For example, additional effectors and regulators of the p53/p21 Cip1 , p16 Ink4a /Rb as well as other potential pathways that induce senescence such as p15 and p27 have been implicated as potential markers to identify senescent cells . In addition, among the first assay biomarkers of cellular senescence was senescence-associated β-galactosidase (SA-β-Gal) , which at pH 6.0 measures endogenous lysosomal β-galactosidase staining that tends to be higher in senescent cells. However, SA-β-Gal activity as a senescence biomarker can be problematic in some tissues, such as bone, due to high background staining and potential false-positive staining in macrophages and osteoclasts . Therefore, as noted above, the combination of more specific markers is recommended. Perhaps the most robust senescent cell biomarker identified to date is referred to as telomere-associated foci (or “ TAF ” ), which are sites of DNA damage co-localized with telomeres.
In addition to TAF-positive nuclei, the cellular senescence phenotype is associated with profound changes in chromatin organization. Altered heterochromatin can result in the formation of senescence-associated heterochromatin foci (SAHFs) that have been shown to accumulate in senescent cells . For example, the presence of SAHFs has been shown by distinct bright DNA foci on DAPI-stained senescent human cells compared to normal human cells . However, the inability to trace SAHFs in models of normal and accelerated aging may render this approach as a less consistent marker of cellular senescence . Another perhaps more reliable marker of senescence based on altered heterochromatin organization that has been more widely reported in both human and preclinical animal models is senescence-associated distension of satellites (or “ SADS ”), which is a measure of the nuclear decondensation of peri-centromeric satellite heterochromatin found in senescent cells . Although these approaches highlight some of the well-established methods to detect senescence, biomarker expression can vary among senescent cell populations, across species and based on the senescence inducing stimuli, which underscores the need to characterize senescence based on a combination of biomarkers.
Identification of senescent cells in old bone
In order to identify the cell type(s) within the bone microenvironment that become senescent in old age, our group studied female and male young (6-month-old) and old (24-month-old) C57BL/6 wild-type (WT) mice from which we isolated highly enriched populations of osteoblast progenitors, osteoblasts, osteocytes, myeloid cells, as well as B- and T-cells. These populations were isolated from either bone itself (using liberase to digest the sample to release osteoblasts and enrich the remaining sample for osteocytes) or from bone marrow using magnetic-activated cell sorting (MACS) based on novel approaches described in our publication . Our data revealed a significant increase in mRNA expression of senescence effector genes, most notably p16 Ink4a , but also in some cases p21 Cip1 and p53 , in the various cell populations of old as compared to young mice. More specifically, p16 Ink4a mRNA expression increased ~ 5–10-fold with aging in each of the cell types isolated in both female and male mice, whereas p21 Cip1 and p53 levels increased to varying degrees in osteocytes and myeloid cells . Our finding of increased senescence with aging in osteocytes was supported by the presence of a significantly higher percentage of in vivo SADS in osteocytes of bone cortices from old relative to young mice . In addition, we found that the percentage of TAF-positive cells and the number of TAFs per osteocyte were both significantly higher in primary osteocytes isolated from older as compared to young mice . Corresponding with these age-related increase in senescence biomarkers, was upregulation of the SASP with aging that was assessed based on an a priori panel of 36 SASP genes identified from the literature . Most notably was the significant upregulation of SASP factors with old age in osteocytes (23 out of 36 SASP genes queried) and myeloid cells (26 out of 36 SASP genes queried) . We also determined that these findings of an increased senescence burden with aging in old mice were also applicable to humans as demonstrated by increased mRNA levels of p16 Ink4a and p21 Cip1 and SASP components (12 out of 36 genes queried) in posterior iliac crest needle bone biopsies isolated from older postmenopausal women relative to younger women . Therefore, senescent cells are present at the time and location of age-related bone loss in both mice and humans.
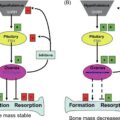
Consistent with findings from our young versus old mouse study , Piemontese and colleagues found an increase in osteocyte senescence, including higher p16 Ink4a mRNA levels and γH2A.X in osteocyte-enriched bone samples isolated from old as compared to young mice. In addition, they identified a potential link between increased osteocyte senescence and higher levels of receptor activator of nuclear factor kappa-B ligand (RANKL—an essential osteoclast differentiation factor) that both correlated with greater endocortical bone resorption and increased cortical porosity with aging . Thus, based on the collective data in mice, it appears that at least a subset of senescent osteocytes accumulate in old bone where potentially through their detrimental pro-inflammatory SASP they accelerate bone resorption in the setting of aging.
Accelerated cellular senescence and skeletal fragility in mice with type 2 diabetes (T2D)
An increased senescent cell burden has also been shown to be found in conditions of accelerated aging attributed to obesity and metabolic dysfunction, including preclinical models of type 2 diabetes (T2D). For example, in response to high-fat diet (HFD) feeding, senescent cells have been shown to accumulate prematurely in obese young adult mice in several tissues, such as adipose , brain , liver , and pancreatic β cells , thus contributing to metabolic dysfunction in these animals . Moreover, Aguayo-Mazzucato et al. recently showed that increased insulin resistance in mice with T2D is associated with pancreatic β-cell senescence and acquisition of the SASP in β-cells, while removal of these senescent cells using either genic INK-ATTAC or senolytic (D + Q) approaches in these mice with metabolic dysfunction improved glucose metabolism as well as insulin secretion, and reduced expression of senescence and SASP markers in pancreatic β-cells.
Evidence from several studies in humans has established higher fracture risk in individuals with T2D despite a normal to higher bone mineral density (BMD) . Therefore, we hypothesized that senescent cells may represent an underlying mechanism at the nexus of skeletal fragility and T2D . Thus, in order to begin to investigate whether bone-resident senescent cells accumulate prematurely in the setting of T2D, our group subjected young adult mice to HFD and a single relatively low dose of streptozotocin (STZ) to induce obesity and adult-onset T2D—i.e., the HFD/STZ mouse model that is commonly used throughout the diabetes field . Consistent with previous studies, we showed that these mice display several key features of T2D such as increased body weight, fat mass, consistently higher glucose (≥ 250 mg/dL) and HbA1c levels, insulin resistance, and decreased pancreatic β-cell area . T2D mice also displayed deteriorated bone quality (assessed by longitudinal micro-CT scanning), including decreased trabecular bone volume fraction (BV/TV), cortical vBMD and thickness as well as micro-finite element analysis (μFEA)-derived bone strength in comparison to control mice . Along with these detrimental changes in bone microarchitecture, directly measured biomechanical bone strength and material properties such as failure load, ultimate stress, and stiffness were significantly diminished in T2D as compared to control mice . Skeletal histomorphometric and serum bone turnover analyses revealed higher bone resorption (osteoclast numbers and cross-linked C-telopeptide of type I collagen [CTx] levels) and lower bone formation rates in T2D mice relative to controls . Additional analyses of osteocyte-enriched bone samples from these mice demonstrated increased mRNA expression of the cellular senescence markers, p16 Ink4a and p21 Cip1 , as well as SASP factors—most notably Mmp’s ( Mmp3 , Mmp9 , Mmp12 , and Mmp13 ) and NFκB . In addition, mice with T2D displayed a significantly higher proportion of senescent osteocytes in vivo in bone cortices as characterized by increased SADS- and TAF-positive staining . These data thus provide compelling evidence for accelerated osteocyte senescence and skeletal fragility in young adult obese mice with T2D.
Effects of reducing the senescent cell burden on bone
Our group has demonstrated that elimination of senescent cells in old INK-ATTAC mice using AP20187 (a synthetic drug that activates the ATTAC suicide transgene and thus apoptosis of p16 Ink4a -positive senescent cells) effectively eliminates senescent osteocytes as well as senescent preadipocytes in old AP20187-treated as compared to age-matched vehicle-treated control mice . Furthermore, the older mice treated with AP20187 displayed better cortical and trabecular bone microarchitecture and improved biomechanical bone strength properties compared to their age-matched vehicle-treated counterparts . Elimination of senescent cells caused a reduction in osteoclast numbers and decreased levels of bone resorption marker—CTX; whereas, senescent cell elimination increased osteoblast numbers and improved bone formation rates on endocortical surfaces assessed by histomorphometry .
As an alternative to the transgenetic INK-ATTAC approach and one potentially more adaptable to human translation, we randomized old WT mice to either intermittent senolytic (D + Q) therapy or vehicle treatments over the course of 4 months. Similar to the old INKA-TTAC mice treated with AP20187, old mice treated with the combination of D + Q displayed better bone microarchitecture and strength and had lower levels of bone resorption along with improved bone formation rates on endocortical surfaces . It should be noted, however, that removal of senescent osteoclast progenitors using ganciclovir in aged p16-3MR mice was not sufficient to alleviate age-related bone loss, perhaps because senescent osteocytes were not eliminated in this model .
As mentioned earlier, senescence-related DDR activation can also occur following high doses of ionizing radiation and chemotherapy, limiting the capacity of the immune system to clear senescent cells from the body, even earlier in life at younger ages. However, both radiation and chemotherapy are used clinically to treat patients with cancer. Although both strategies are effective in limiting the spread of malignant cells, a byproduct is the premature, accelerated accumulation of senescent cells. To examine the bystander effects of senescent cell accumulation on bone loss in the setting of radiation, Chandra et al. performed single-limb (at the femoral metaphysis) focal radiation therapy (FRT) on young adult mice. They found a 4.4-fold increase in SA-β-Gal-positive osteoblasts in the radiated femur versus the contralateral nonradiated femur of these mice . This was accompanied by a 4.7-fold increase in TAF-positive osteocytes and a 3.7-fold increase in TAF-positive osteoblasts in the radiated femur as compared to the contralateral femur . Increased mRNA expression of senescent markers, p16 Ink4a , p21 Cip1 , p15 , and p27 , and SASP components (18 out of 46 genes queried) were also found in the radiated femur compared to the nonradiated contralateral limb . This increased senescence burden in radiated bone was accompanied by deterioration of trabecular bone microarchitecture, and treatment of radiated mice with senolytics (D + Q) decreased bone mRNA expression of p16 Ink4a , p21 Cip1 , and SASP genes (e.g., Ccl-2 , Ccl-4 , Cxcl-9 , Cxcl-13 , IL-10 , IL-18 , and Mmp2 ) as compared to vehicle-treated mice . Removal of senescent cells restored trabecular bone parameters, increased osteoblast numbers and levels of the bone formation marker, P1NP, and decreased the numbers of bone marrow adipocytes, compared to the vehicle-treated mice also receiving FRT . Therefore, targeting senescent cells with senolytics may represent an effective strategy to preserve bone in cancer patients receiving radiotherapy.
In addition to radiotherapy, chemotherapy-induced bone loss has also been hypothesized to be at least partially a consequence of increased senescent cell accumulation. To test this hypothesis, Yao et al. recently examined the effects of doxorubicin (DOXO), a chemotherapeutic agent used for the treatment of early and advanced-stage breast cancer, on bone parameters and cellular senescence in the bone microenvironment of young adult mice. As anticipated, mice given DOXO developed a lower bone mass phenotype affecting both trabecular and cortical bone parameters as compared to vehicle-treated mice . Female DOXO-treated mice also had a greater reduction in bone parameters compared to ovariectomized (OVX) mice thus establishing that chemotherapy induces bone loss independent of estrogen deficiency . Similar results were found with another chemotherapeutic agent, paclitaxel . Mice treated with DOXO also displayed higher bone mRNA expression of p16 Ink4a and lower levels of HMGB1 as compared to the vehicle-treated mice , which are both indicative of a higher senescent cell burden in bone. Additionally, DOXO-treated mice displayed upregulated SASP components in bone samples such as IL-6 , Ccl3 , MCSF , and MMP12 relative to vehicle-treated mice . By contrast, similar senescence- and SASP-associated changes were not observed in OVX mice implying that lack of estrogen by itself is not sufficient to cause cellular senescence . Treatment of DOXO-induced INK-ATTAC mice using AP20187 resulted in decreased p16 Ink4a and IL-6 and increased HMGB1 levels, and a concomitant increase in cortical and trabecular bone parameters, bone formation rates, and decreased osteoclast numbers as compared to the vehicle group . Therefore, consist with clearance of senescent cells in old mice to prevent age-related bone loss , genetic clearance of p16 Ink4a -positive cells may represent a novel strategy to alleviate cancer-induced bone loss.
Cellular senescence and aged skeletal muscle
Although heterogeneous in nature, mammalian skeletal muscle is predominantly a mitotically stable tissue with limited turnover yet there is a small population of quiescent muscle stem cells (i.e., satellite cells) that have important biological roles and have been implicated in cellular senescence. Traumatic muscle injury or genetic defects, such as muscular dystrophies, can activate quiescent satellite cells that then undergo dynamic interactions to repair damaged myofibers. Proliferation and differentiation of activated satellite cells can result in their fusion with the damaged myofibers and with other satellite cells to initiate a repair response of the damaged skeletal muscle fibers or can give rise to new fibers.
Satellite cells can self-renew to maintain the skeletal muscle stem cell pool by dividing into daughter stem cells and returning to quiescence following muscle repair thereby maintaining skeletal muscle regenerative capacity . However, satellite cell regenerative capabilities have been shown to decline with aging, and the molecular mechanisms regulating quiescence in satellite cells have been linked to the regulation of senescence-associated genes . In particular, quiescent satellite cells in sarcopenic myofibers of geriatric mice display hallmarks of sarcopenia, including impaired activation in response to injury and reduced regenerative capacity . In old age, these cells tend to break away from their state of quiescence and enter into a presenescence state under homoeostatic conditions which is evidenced by their increased p16 Ink4a mRNA expression compared to younger mice . This quiescence to senescence shift of satellite cells has been attributed to the accumulation of genomic damage with advancing age, which can be reversed by repression of p16 Ink4a using short-hairpin RNA (shRNA) to thereby decreasing p16 Ink4a levels and increase the activation rate and self-renewal capacity of satellite cells following muscle injury . Similar to the findings in mice, assessment of human satellite cells isolated from older individuals with sarcopenia (aged ~ 75 years) showed significantly increased p16 Ink4a mRNA levels, SA-β-Gal staining, and a dysregulated p16-Rb pathway as compared to satellite cells isolated from younger human subjects . Collectively, these data provide evidence that in response to age-related stress, quiescent muscle satellite cells can enter a state of cellular senescence that limits their resilience and regenerative capacity, thus contributing to aging, frailty, and sarcopenia.
Causal roles of senescent cells in physical dysfunction and frailty
The link between natural aging and declining physical function and increased frailty is clear. In addition to maintaining skeletal muscle regeneration, several studies have shown reduced physical function and lifespan, most notably in mouse models of cancer therapy or accelerated aging, characterized with an increased senescent cell burden. For example, Zhu and colleagues reported that clearance of senescent cells using senolytics, D + Q, improved physical function in mice by alleviating impairment induced by radiation exposure. Indeed, months after radiation, mice that were treated with senolytics had the ability to exercise longer on a treadmill (i.e., more time, distance, as well as total work) and maintained their greater endurance capacity for at least 7 months following senolytic therapy as compared to the vehicle-treated mice . Furthermore, senolytic therapy resulted in decreased expression of p16 Ink4a in skeletal muscle and a reduced proportion of SA-β-Gal-positive cells in inguinal fat , suggesting that the benefits on physical function where potentially mediated through senescent cell clearance in skeletal muscle and adipose tissue depots.
As an extension to this study, Xu and colleagues more recently investigated the causal roles of cellular senescence in mediating physical dysfunction and frailty using multiple approaches. In the first of a series of elegant experiments, they transplanted a small number of senescent preadipocytes intraperitoneally into young adult WT mice, which after only 1 month caused persistent physical dysfunction, including decreased maximal walking speed, shorter hanging endurance, and reduced grip strength as compared to control mice transplanted with control, nonsenescent preadipocytes . Furthermore, an increased senescence burden (e.g., higher p16 Ink4a expression, increased TAF-positive cells, and SA-β-Gal-positive cells) was observed locally in visceral adipose tissue (near the injection site) in mice transplanted with senescent cells even after the transplanted cells (which were traced in vivo using a luciferase reporter) were cleared from the body of these animals . In addition, the spread of senescence was noted in tissues distant from the site of injection (e.g., skeletal muscle) as evidenced by increased expression of p16 Ink4a and pro-inflammatory SASP cytokines such as TNFα and IL-6, thus demonstrating the systemic spread of cellular senescence to the host’s tissues . Transplantation of even fewer senescent cells into older mice was sufficient to accelerate physical dysfunction and frailty in these animals and significantly increased their mortality . In complementary experiments, clearance of senescent cells in normal, chronologically aged WT mice by periodically administering senolytics, D + Q, to these animals alleviated physical dysfunction and aspects of frailty by reducing senescent cell abundance; importantly, senescent cell clearance in these animals also extended posttreatment survival (by 36%) while simultaneously compressing morbidity . Interestingly, senescent cell clearance was also observed following administration of D + Q to surgically excised ex vivo adipose tissue explants from obese humans , pointing to the translational relevance of this strategy to target cellular senescence to potentially improve physical function and alleviate aspects of frailty with aging and in the setting of metabolic dysfunction. Whether these or similar approaches are effective in ameliorating sarcopenia remains to be established.
Further potential translational evidence for the roles of cellular senescence in driving physical dysfunction with aging comes from studies performed in humans that have reported an accumulation of p16 Ink4a -positive cells in thigh adipose tissue biopsies obtained from older as compared to younger subjects . Furthermore, the elevated proportion of p16 Ink4a -positive cells in thigh adipose tissue correlated negatively with measures of physical performance such as grip strength, gait speed on 4-m course and self-perceived mobility, even after controlling for potentially confounding factors including age and measures of body composition . Although these studies provide initial evidence for the association between a greater senescent cell burden and physical dysfunction in humans, additional larger prospective studies are warranted.
Senolytics in clinical trials
Clinical trials investigating the safety and efficacy of senolytics in humans are currently underway, and results from the first studies were recently reported. For example, in the first-in-human pilot study of D + Q conducted on 14 patients with idiopathic pulmonary fibrosis (IPF), a chronic, rapidly progressing fibrotic lung disease linked to cellular senescence , Justice and colleagues administered D + Q intermittently in the form of 9 doses over the course of 3 weeks to 14 IPF patients. Although this study lacked a placebo-controlled group, a significant improvement was observed in these patients in 6-min walk distance, 4-m gait speed, chair stand time, and short physical performance battery scores in response to intermittent senolytic therapy . There was also a trend for a decrease in SASP factors within the circulation, although changes did not reach statistical significance potentially due to the relatively small sample size . Nevertheless, given the modest improvements in physical function and that D + Q was found to be well-tolerated and safe, this study supports the need for additional senolytic trials. One such ongoing phase 1, open-label, trial in patients with diabetic kidney disease has reported interim results . Indeed, Hickson and colleagues administered the combination of D + Q to patients with diabetic kidney disease for three consecutive days and then examined the in vivo senescent cell burden in these subjects 11 days following the final dose. Consistent with data form preclinical studies, a significant reduction in p16 Ink4a – and p21 Cip1 -positive cells as well as significantly reduced SA-β-Gal staining was observed in subcutaneous abdominal tissue samples of D + Q-treated subjects with similar findings observed in skin biopsies . In addition, several SASP factors including IL-1α, -2, -6, and -9, and MMP-2, -9, and -12 were significant reduced in the circulation of subjects treated with D + Q . Although again not placebo-controlled and preliminary, this study provides initial “ proof-of-concept ” evidence that senolytic therapy can reduce the in vivo cellular senescence burden in humans, as observed in mice. It should be noted that multiple other placebo-controlled, randomized controlled clinical trials involving senolytics are currently underway as more data on safety, tolerability, side effects, and efficacy are needed in humans. Furthermore, additional carefully monitored trials in humans with other conditions, such as elderly subjects with age-related bone loss, will be initiating soon.
Conclusion
In summary, there is hope that the enormous burden of age-related co-morbidities, including osteosarcopenia, can be mitigated by therapeutically targeting fundamental mechanisms of aging, such as cellular senescence. Indeed, mounting evidence, predominantly in mice (as summarized in Table 1 ), points to reducing the senescent cell burden to prevent or delay age-related bone loss and physical dysfunction as well as aspects of frailty in old age. Additional clinical studies and trials are needed to establish whether these exciting preclinical findings can be translated to humans.