Chapter 10 Cell-mediated Cytotoxicity
• Cell-mediated cytoxicity is an essential defence against intracellular pathogens, including viruses, some bacteria and some parasites.
• CTLs and NK cells are the lymphoid effectors of cytotoxicity. Most CTLs are CD8+ and respond to non-self antigens presented on MHC class I molecules. Some virally infected and cancerous cells try to evade the CTL response by downregulating MHC class I. NK cells recognize these MHC class I negative targets.
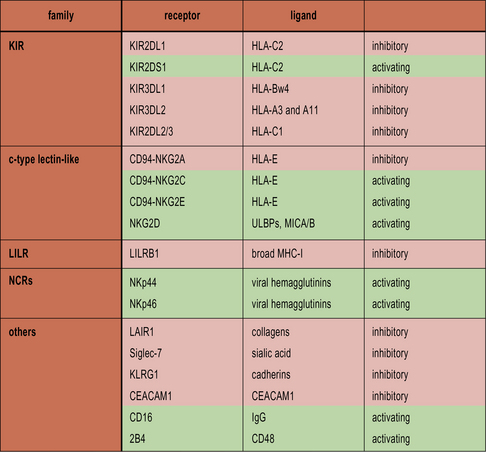
• NK cells recognize cells that fail to express MHC class I. NK cells express a variety of inhibitory receptors that recognize MHC class I molecules. When these receptors are not engaged, the NK cell is activated. Killer Immunoglobulin-like Receptors (KIRs) recognize classical MHC class I molecules. CD94 interacts with HLA-E. LILRB1 recognizes a wide range of class I molecules.
• Cancerous and virally infected cells express ligands for the activating receptor NKG2D. Stressed cells, including cancerous and virally infected cells, upregulate ULBP1–3, MICA and MICB, which are ligands for NKG2D. This results in NK cell activation.
• NK cells can also mediate ADCC.
• The balance of inhibitory and activating signals determines NK cell activation.
• Cytotoxicity is effected by direct cellular interactions, granule exocytosis and cytokine production. Fas ligand and TNF can induce apoptosis in the target cell. Granules containing perforin and granzymes are also released. Perforin forms a pore in the cell membrane, allowing granzymes access to the cytosol. Granzymes trigger the cell’s intrinsic apoptosis pathways.
• Macrophages, neutrophils and eosinophils are non-lymphoid cytotoxic effectors. Macrophages and neutrophils usually destroy pathogens by phagocytosis, but can also sometimes release the contents of their granules into the extracellular environment. Eosinophils release cytotoxic granules in response to antibody-coated cells.
Cytotoxic lymphocytes
Several types of cells have cytotoxic potential, including:
CTLs and NK cells mediate cytotoxicity
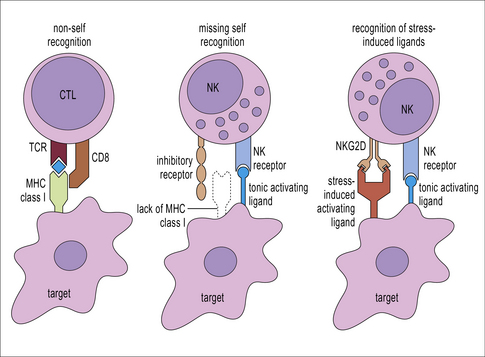
T cells and NK cells belong to the lymphoid lineage, and are more closely related to each other than they are to B cells. CTLs and NK cells both induce apoptosis in their targets by production of TNF family molecules, cytokines and cytotoxic granules, but they recognize their targets in different ways. CTLs recognize foreign antigens being presented on MHC class I, whereas NK cells respond to cells that fail to express class I. NK cells are also able to recognize stressed or antibody-coated targets directly (Fig. 10.1).
CTLs recognize antigen presented on MHC class I molecules
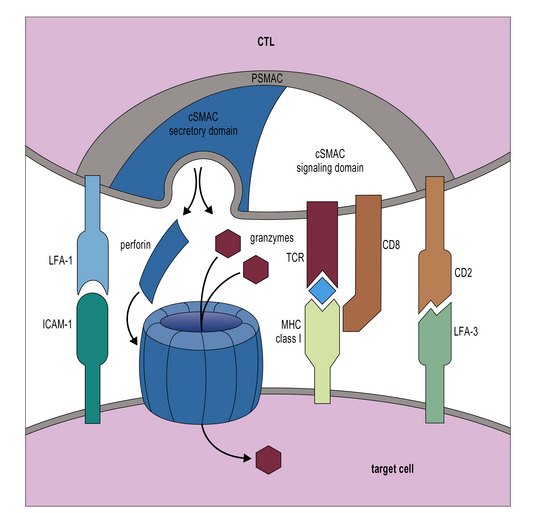
Additional interactions may be required to stabilize the bond between a CTL and its target. Like CD4+ T cells, CD8+ CTLs form an immunological synapse with their target. Signaling molecules including the TCR and CD3 are found in the central zone of the supra-molecular activation cluster (cSMAC), while adhesion molecules segregate in the peripheral zone (pSMAC). In contrast to CD4+ T cells, the cSMAC of CTLs and NK cells is divided into signaling and secretory domains (Fig. 10.2). After signaling has occurred, the microtubule organizing center polarizes towards the synapse, directing cytotoxic granules to the secretory domain of the cSMAC. Early CTL signaling occurs within ten seconds of cell–cell contact and granule release follows some two minutes later.
Not all NK cells mediate cytotoxicity
• 90% of blood NK cells are CD56low (i.e. they have low expression of CD56). These cells contain cytotoxic granules and are effective killers;
• 10% of blood NK cells are CD56hi (i.e. they express high levels of CD56). These cells do not contain cytotoxic granules, but can respond to target cells by producing the TH1 cytokine IFNγ;
• NK cells present in the lymph nodes, liver, and lungs are also less cytotoxic than CD56low blood NK cells;
• NK cells found in the uterus do contain cytotoxic granules, but do not degranulate in response to target cells. Their function is likely to be the production of angiogenic factors and mediation of placental implantation;
• a recently described subset of NK-like cells found in MALT are not cytotoxic but do produce IL-22, which is important for mucosal integrity.![]()
NK cell development
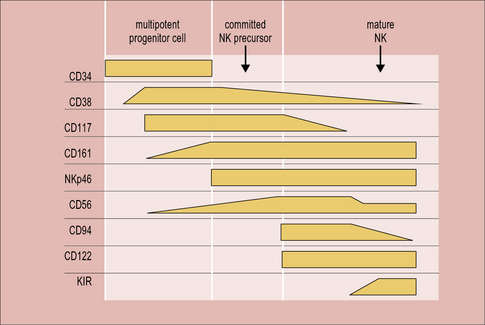
In mice, the bone marrow is essential for the production of NK cells, and a complete pathway of NK development has been described at this location. Human bone marrow does contain CD34+ hematopoietic progenitor cells that have the potential to differentiate into NK cells, but NK-committed progenitor cells have not been identified here. On the other hand, a complete scheme of NK cell development has been described in human, but not mouse, secondary lymphoid tissue. Circulating CD34+ cells are thought to be recruited from the blood to the lymph nodes, where they progress through an NK-committed immature stage to give rise to CD94+CD56hi mature NK cells, capable of effector functions (Fig. 10.w1). CD56hi cells can further develop into CD56low cells, which express KIRs and are more cytotoxic than CD56hi cells. A thymic pathway of NK cell development has also been described in both humans and mice. However, this pathway is clearly not required for NK cell production as athymic individuals have normal NK cell numbers and function.
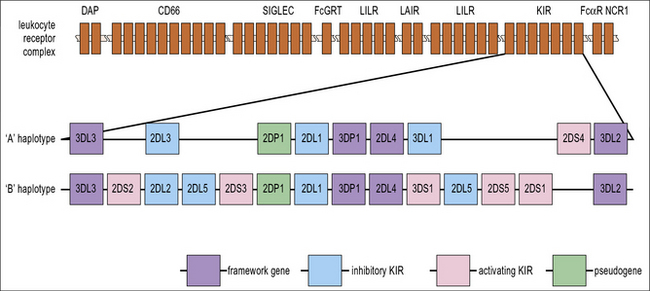
For the acquisition of KIRs, which occurs at the final stage of development, as CD56hi NK cells become CD56low, contact with stromal cells is once again required. Defective Ly49 expression by NK cells in the Tyro/Axl/Mer triple knockout mouse suggests that this may be partially because there is a requirement for an interaction between Protein S or Gas6 on stroma and one of their receptors (Tyro, Axl or Mer) on developing NK cells. Stromal MHC class I molecules are also likely to be important for acquisition of KIR. Several of the genes that are required for immune recognition by NK cells are grouped together on the leukocyte receptor complex on the long arm of chromosome 19 (Fig. 10.w2).
NK cell receptors
NK cells recognize cells that fail to express MHC class I
NK cells express inhibitory receptors that bind to MHC class I molecules. When they encounter a target cell that is not expressing MHC class I, this inhibitory signal is lost and tonic activating signals cause the NK cell to degranulate or produce cytokines in response to the target cell. Interestingly, many of the inhibitory receptors expressed by NK cells also have activating counterparts, many of which recognize the same ligands but with lower affinity. The function of these activating receptors is not known (Fig. 10.3).