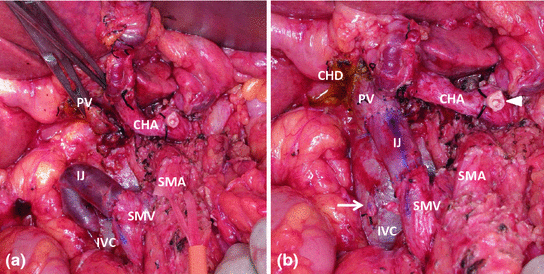
Fig. 29.1
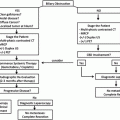
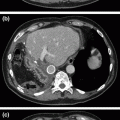
a–c—a demonstrates a CT axial image of a hypoechoic tumor (*) at the bifurcation of the celiac artery into the splenic artery (SA) and common hepatic artery (CHA; red arrows). The blue arrow marks the portal vein. b is a computed tomography coronal image showing the portal vein (cephalad blue arrow) occluded by the tumor (*) and reconstituting into the superior mesenteric vein (lower blue arrow) and its branches caudal to the region of tumor involvement. c is a coronal CT venous reconstruction image showing cavernous transformation of the portal vein with associated venous collaterals (blue arrows). The asterisk marks the tumor location
Diagnosis and Preoperative Management
Patients with newly diagnosed pancreatic cancer are treated by a multidisciplinary team with a combination of chemotherapy, radiation, and surgical resection (when possible) [1–3]. Venous resection and reconstruction during pancreatectomy are now considered standard of care for pancreatic cancer, as supported by the consensus statement published by the American Hepato-Pancreato-Biliary Association/Society of Surgical Oncology in 2009 [4]. Venous resection during pancreatectomy is done when the only obstacle for a complete R0 resection of the tumor is the inability to separate the tumor from the attached venous segment, presuming there is an adequate proximal and distal target available for reconstruction [5]. Although consensus statements such as the one referenced above imply that venous resection and reconstruction (at the time of pancreaticoduodenectomy or total pancreatectomy) has become somewhat routine, we would argue that such operations are of significant complexity, and require years of experience to both be performed safely and to result in a complete gross resection of the tumor.
The multimodality management of pancreatic cancer is founded on the initial staging of the tumor, which is based on anatomic tumor–vessel relationships. We have developed a CT-based staging algorithm for pancreatic cancer whereby we classify tumors as resectable, borderline resectable, locally advanced, or metastatic [2, 6, 7]. Tumors with more than 50% abutment of the SMV-PV are considered borderline resectable. Therefore, patients who require mesocaval shunting and segmental venous resection with interposition grafting have, by definition, borderline resectable disease at a minimum.
Experienced interventional endoscopists are a key part of the multidisciplinary team, as a tissue diagnosis is required prior to initiation of neoadjuvant therapy. EUS/FNA of the tumor is performed with an on-site cytopathologist specializing in pancreatic cytopathology. This facilitates a prompt, usually same setting, tissue diagnosis. Durable metallic endobiliary stents are inserted in patients with biliary obstruction prior to the initiation of neoadjuvant therapy [11].
After diagnosis and accurate staging, patients with borderline resectable pancreatic cancer receive neoadjuvant therapy. National guidelines support the use of neoadjuvant chemotherapy with/without chemoradiation in patients with borderline resectable disease [1, 8, 9]. Neoadjuvant therapy has been shown to increase the rate of R0 resections and reduce the number of patients with positive lymph nodes, both of which positively impact survival [8]. Neoadjuvant therapy also allows clinicians a window of time to assess tumor biology; thus, only patients with stable or responding disease at restaging are offered surgical resection directed at the primary tumor [1, 10].
Stage-specific treatment plans are assigned to every patient treated in our pancreatic cancer program. Serial restaging and assessment of treatment response are done at regular intervals following completion of each modality of therapy and again prior to surgery. Restaging includes assessment of three parameters: clinical, biochemical, and radiographic response to neoadjuvant treatment [2, 9]. Clinical response is based on performance status and symptoms (ECOG ≤ 1, improvement in pain). The biochemical response is based on serum tumor markers; we usually obtain a serum level of CA 19-9 at diagnosis once the bilirubin level has normalized, and then obtain serial levels at every restaging evaluation. Disease progression should be suspected when the CA 19-9 increases even in the absence of clinical or radiographic signs of disease progression. Our group and others have published the positive prognostic impact of a decline in Ca 19-9 to normal levels following neoadjuvant therapy [12, 13]. The radiographic response is also assessed at the end of every phase of the neoadjuvant therapy. This assessment is made accurate by the inclusion of experienced diagnostic radiologists and a weekly multidisciplinary pancreas tumor conference. Preoperative restaging scans are obtained no greater than 30 days prior to surgery, and normally within 2 weeks of the date of operation. Tumor–vessel relationships and the plan for vascular resection, if needed, are assessed and discussed one final time before surgery. It is important to note that the tumor–vessel relationship usually remains unchanged even when the primary tumor responds to therapy (gets smaller). Based on these clinical, biochemical, and radiographic assessments, patients are classified as having had a response to treatment, stable disease, or progressive disease [2, 7].
All patients with cavernous transformation of the PV or partial/complete occlusion of the PV or SMV are placed on a prophylactic dose of low-molecular-weight heparin at the time of diagnosis. Patients are medically supported (hydration, growth factor support, antiemetics, nutrition) throughout their neoadjuvant therapy by our multidisciplinary team. A surgical clinic visit is usually scheduled within 2 weeks of surgery to review restaging studies, to obtain medical clearance, and to develop a detailed operative plan. A consent form is signed for diagnostic laparoscopy, pancreatectomy, and possible internal jugular and saphenous vein harvest.
The patient reported herein was seen as a second opinion after completing preoperative systemic chemotherapy and chemoradiation for what was believed to be an unresectable tumor. When referred to our program, initial imaging and all restaging scans were reviewed. The tumor–vessel relationships were stable (unchanged) and there was total occlusion of the SMV-PV confluence with cavernous transformation of the PV forming collaterals around the head and neck of the pancreas. Venous reconstruction was possible due to the presence of proximal (PV) and distal (SMV) targets for segmental venous reconstruction. The pancreatic neck tumor was abutting the celiac axis bifurcation at the level of the SA and the CHA and the SA was encased. Her performance status was adequate (ECOG 0/1), and she was not requiring pain medication.
CA 19-9 had dropped from 216 to 33 units/mL. Consensus review was a favorable clinical and biochemical response and stable imaging findings. She was therefore consented for total pancreatectomy, en bloc splenectomy, and mesocaval shunting utilizing internal jugular vein.
Surgical Management
Patients receive prophylactic antibiotics on induction. Their power port is accessed and we do not usually utilize additional central venous access, in order to preserve the internal jugular veins if a mesocaval shunt/interposition graft is required. The abdomen, left neck, and the groin are widely prepped and a detailed procedure “time out” is completed. A diagnostic laparoscopy is routinely performed to rule out radiologically occult metastatic disease [14]. Pancreatectomy is completed as we have previously published [15]. Cavernous transformation of the PV is caused by gradual occlusion of the PV. This occlusion results in the development of multiple large venous collaterals, allowing for mesenteric venous return to the liver by bypassing the obstruction. This poses a significant risk of hemorrhage if the portal dissection were to be attempted without decompression of the venous collaterals [16]. We have used mesocaval shunting during pancreatectomy to safely address the issue of large portal venous collaterals in the setting of an occluded PV [16, 17]. The SMV is exposed early in the operation, inferior to the neck of the pancreas or caudal to the area of tumor encasement; an area usually free of venous collaterals (Fig. 29.2a) The middle colic vein is usually divided to allow adequate exposure of the SMV. The anterior surface of the IVC is cleared caudal to the renal veins when performing a generous Kocher maneuver. Additional dissection at the root of the mesentery allows identification of the superior mesenteric artery (SMA) as well as the jejunal and ileal branches of the SMV (if the SMV is being approached cephalad to its bifurcation). Exposure of the SMA and SMV caudal to the tumor is critically important. An artery (SMA)-first approach, which involves separation of the pancreatic head and uncinate process from the SMA (before the SMV-PV), is often performed, even if not complete, as it will facilitate exposure of the IVC from the root of mesentery and allow for a bit more room to perform the SMV and the IVC anastomoses [18, 19]. The jejunal branch of the SMV usually courses posterior to the SMA [20]. An anterior jejunal branch allows easier access to the SMV, however it is associated with multiple venous anomalies [5]. When needed for oncologic and/or technical reasons, one of the first-order branches of the SMV may often be ligated without the need for reconstruction. This is acceptable if the caliber of the remaining ileal branch is suitable for reconstruction (at least 1.5 times the diameter of the SMA on the axial images of the CT scan). While anastomosis to the ileal branch of the SMV is quite reliable (as compared to the common trunk of the SMV), anastomosis to the jejunal branch is quite difficult, and should only be attempted in very rare situations by the most experienced surgeons [20]. Bowel ischemia and necrosis will occur if both the ileal and the jejunal branches of the SMV are divided [15].