Cause
In what circumstances can we pass from observed association to a verdict of causation? Upon what basis should we proceed to do so?
— Sir Austin Bradford Hill 1965
KEY WORDS
Web of causation
Aggregate risk studies
Ecological studies
Ecological fallacy
Time-series studies
Multiple time-series studies
Decision analysis
Cost-effectiveness analysis
Cost-benefit analysis
This book has been about three kinds of clinically useful information. One is description, a simple statement of how often things occur, summarized by metrics such as incidence and prevalence, as well as (in the case of diagnostic test performance) sensitivity, specificity, predictive value, and likelihood ratio. Another is prediction, evidence that certain outcomes regularly follow exposures without regard to whether the exposures are independent risk factors, let alone causes. The third is either directly or implicitly about cause and effect. Is a risk factor an independent cause of disease? Does treatment cause patients to get better? Does a prognostic factor cause a different outcome, everything else being equal? This chapter considers cause in greater depth.
Another word for the study of the origination of disease is “etiology,” now commonly used as a synonym for cause, as in “What is the etiology of this disease?” To the extent that the cause of disease is not known, the disease is said to be “idiopathic” or of “unknown etiology.”
There is a longstanding tendency to judge the legitimacy of a causal assertion by whether it makes sense according to beliefs at the time, as the following historical example illustrates.
Example
In 1843, Oliver Wendell Holmes (then professor of anatomy and physiology and later dean of Harvard Medical School), published a study linking hand washing habits by obstetricians and childbed (puerperal) fever, an often-fatal disease following childbirth. (Puerperal fever is now known to be caused by a bacterial infection.) Holmes’s observations led him to conclude that “the disease known as puerperal fever is so far contagious, as to be frequently carried from patient to patient by physicians and nurses (1).”
One response to Holmes’s assertion was that the findings made no sense. “I prefer to attribute them [puerperal fever cases] to accident, or Providence, of which I can form a conception, rather than to contagion of which I cannot form any clear idea, at least as to this particular malady,” wrote Charles Meigs, professor of midwifery and the diseases of women and children at Jefferson Medical College. Around that time, a Hungarian physician, Ignaz Semmelweis, showed that disinfecting physicians’ hands reduced rates of childbed fever, and his studies were also dismissed because he had no generally accepted explanation for his findings. Holmes’s and Semmelweis’s assertions were made decades before pioneering work— by Louis Pasteur, Robert Koch, and Joseph Lister—established the germ theory of disease.
The importance attached to a cause-and-effect relationship “making sense,” usually in terms of a biologic mechanism, is still imbedded in current thinking. For example, in the 1990s, studies showing that eradication of Helicobacter pylori infection prevented peptic ulcer disease were met with skepticism because everyone knew that ulcers of the stomach and duodenum were not an infectious disease. Now, H. pylori infection is recognized as a major cause of this disease.
In this chapter, we review concepts of cause in clinical medicine. We discuss the broader array of evidence, in addition to biologic plausibility, that strengthens or weakens the case that an association represents a cause-and-effect relationship. We also briefly deal with a kind of research design not yet considered in this book: studies in which exposure to a possible cause is known only for groups and not for the individuals in the groups.
BASIC PRINCIPLES
Single Causes
In 1882, 40 years after the Holmes-Meigs confrontation, Koch set forth postulates for determining that an infectious agent is the cause of a disease (Table 12.1). Basic to his approach was the assumption that a particular disease has one cause and that a particular cause results in one disease. This approach helped him to identify for the first time the bacteria causing tuberculosis, diphtheria, typhoid, and other common infectious diseases of his day.
Koch’s postulates contributed greatly to the concept of cause in medicine. Before Koch, it was believed that many different bacteria caused any given disease. The application of his postulates helped bring order out of chaos. They are still useful today. That a unique infectious agent causes a particular infectious disease was the basis for the discovery in 1977 that Legionnaire disease is caused by a gram-negative bacterium; the discovery in the 1980s that a newly identified retrovirus causes AIDS; and the discovery in 2003 that a coronavirus caused an outbreak of severe acute respiratory syndrome (SARS) (2).
Table 12.1 Koch’s Postulates | ||||
|---|---|---|---|---|
|
Multiple Causes
For some diseases, one cause appears to be so dominant that we speak of it as the cause. We say that Mycobacterium tuberculosis causes tuberculosis or that an abnormal gene coding for the metabolism of phenylalanine, an amino acid, causes phenylketonuria. We may skip past the fact that tuberculosis is also caused by host and environmental factors and that the disease phenylketonuria develops because there is phenylalanine in the diet.
More often, however, various causes make a more balanced contribution to the occurrence of disease such that no one stands out. The underlying assumption of Koch’s postulates, one cause— one disease, is too simplified. Smoking causes lung cancer, coronary artery disease, chronic obstructive pulmonary disease, and skin wrinkles. Coronary artery disease has multiple causes, including cigarette smoking, hypertension, hypercholesterolemia, diabetes, inflammation, and heredity. Specific parasites cause malaria, but only if the mosquito vectors can breed, become infected, and bite people, and those people are not taking antimalarial drugs or are unable to control the infection on their own.
When many factors act together, it has been called the “web of causation” (3). A causal web is well understood in chronic degenerative diseases such as cardiovascular disease and cancer, but it is also the basis for infectious diseases, where the presence of a microbe is a necessary but not sufficient cause of disease. AIDS cannot occur without exposure to HIV, but exposure to the virus does not necessarily result in disease. For example, exposure to HIV rarely results in seroconversion after needlesticks (about 3/1,000) because the virus is not nearly as infectious as, say, the hepatitis B virus. Similarly, not everyone exposed to tuberculosis—in Koch’s day or now—becomes infected.
When multiple causes act together, the resulting risk may be greater or less than would be expected by simply combining the effects of the separate causes. That is, they interact—there is effect modification. Figure 12.1 shows the 10-year risk of cardiovascular disease in a 60-year-old man with no prior history of cardiovascular disease according to the presence or absence of several common risk factors. The risk is greater than the sum of the effects of each individual risk factor. The effect of low HDL is more in the presence of elevated total cholesterol, the effect
of smoking is more in the presence of both elevated total cholesterol and low HDL, and so on. The consequence of exposure to each new risk factor is affected by exposure to the others, an example of effect modification. Age and sex are also risk factors and interact with the others (not shown).
of smoking is more in the presence of both elevated total cholesterol and low HDL, and so on. The consequence of exposure to each new risk factor is affected by exposure to the others, an example of effect modification. Age and sex are also risk factors and interact with the others (not shown).
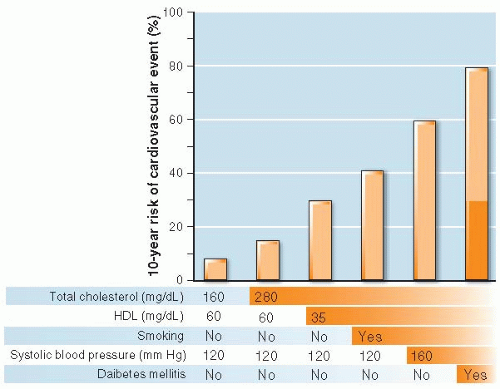 Figure 12.1 ▪ The interaction of multiple risk factors for cardiovascular disease. Ten-year cardiovascular risk (%) for a 60-year-old man with no risk factors (left bar) and with the successive addition of five risk factors (bars to the right). Each risk factor alone adds relatively little (several percent) to risk whereas adding them to each other increases risk almost 10-fold, far more than the sum of the individual risk factors acting independently, which is shown by the shaded area of the right-hand bar. (Data from The Framingham Risk Calculator in UpToDate, Waltham, MA according to formulae in D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care. The Framingham Heart Study. Circulation 2008;117(6):743-753.) |
When multiple causative factors are present and interact, it may be possible to make a substantial impact on a patient’s health by changing just one or a few of them. In the previous example, treating hypertension and elevated serum cholesterol can substantially lower the risk of developing cardiovascular disease, even if the other risk factors are unchanged.
By and large, clinicians are more interested in treatable or reversible causes than immutable ones. For example, when it comes to cardiovascular disease, age and sex cannot be changed—they have to be taken as a given. On the other hand, smoking, blood pressure, and serum cholesterol can be changed. Therefore, even though risks related to age and sex are at least as big as for risk factors as the others and are taken into account when estimating cardiovascular risk, they do not offer a target for prevention or treatment.
Proximity of Cause to Effect
When biomedical scientists study cause, they usually search for the underlying pathogenetic mechanism or final common pathway of disease. Sickle cell anemia is an example. In simplified form, pathogenesis involves a gene coded for abnormal hemoglobin that polymerizes in low-oxygen environments (the capillaries of some tissues), resulting in deformed red cells, causing anemia as they are destroyed, and occluding vessels, causing attacks of ischemia with pain and tissue destruction.
Disease is also determined by less specific, more remote causes (risk factors) such as behavior and environments. These factors may have large effects on disease rates. For example, a large proportion of cardiovascular and cancer deaths in the United States can be traced to behavioral and environmental factors such as cigarette smoking, diet, and lack of exercise; AIDS is primarily spread through unsafe sexual behaviors and shared needles; and deaths from violence and unintended injuries are rooted in social conditions, access to guns, intoxication while driving, and seatbelt use.
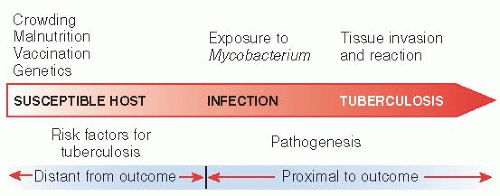
Figure 12.2 shows how both risk factors and pathogenesis of tuberculosis—distant and proximal causes—lead on a continuum to the disease. Exposure to M. tuberculosis depends on the host’s environment: close proximity to active cases. Infection depends on host susceptibility, which can be increased by malnutrition, decreased by vaccination, and altered by genetic endowment. Whether infection progresses to disease depends on these factors and others, such as immunocompetence, which can be compromised by HIV infection and age. Finally, active infection may be cured by antibiotic treatment.
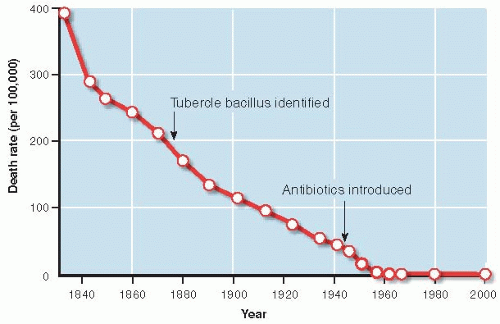
Clinicians may be so intent on pathogenesis that they underestimate the importance of more remote causes of disease. In the case of tuberculosis, social and economic improvements influencing host susceptibility, such as less crowded living space and better nutrition, appear to have played a more prominent role in the decline in tuberculosis rates in developed countries than treatments. Figure 12.3 shows that the death rate from tuberculosis in England and Wales dropped dramatically before the tubercle bacillus was identified and a century before the first effective antibiotics were introduced in the 1950s.
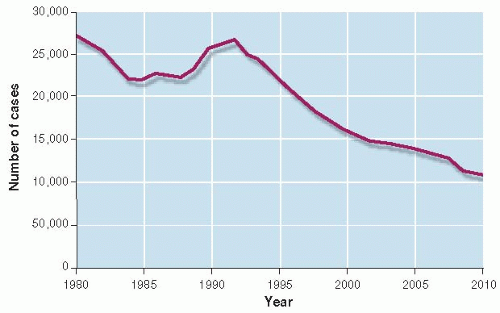
The web of causation is continually changing, even for old diseases. Between 1985 and 1992, the number of tuberculosis cases in the United States, which had been falling for a century, began to increase (Fig. 12.4) (4). Why did this happen? There had been an influx of immigrants from countries with high rates of tuberculosis. The AIDS epidemic produced more people with a weakened immune system, making them more susceptible to infection with M. tuberculosis. When infected, their bodies allowed massive multiplication of the bacterium, making them more infectious. Rapid multiplication, especially in patients
who did not follow prescribed drug regimens, favored the development of multidrug resistant strains. People who were more likely to have both AIDS and tuberculosis—the socially disadvantaged, intravenous drug users, and prisoners—were developing multidrug resistant disease and exposing others in the population to a difficult-to-treat strain. The interplay of environment, behavior, and molecular biology combined to reverse a declining trend in tuberculosis. To combat the new epidemic of tuberculosis, the public health infrastructure was rebuilt. Multidrug regimens (biologic efforts) and directly observing therapy to ensure compliance (behavioral efforts) were initiated and the rate of tuberculosis began to decline again.
who did not follow prescribed drug regimens, favored the development of multidrug resistant strains. People who were more likely to have both AIDS and tuberculosis—the socially disadvantaged, intravenous drug users, and prisoners—were developing multidrug resistant disease and exposing others in the population to a difficult-to-treat strain. The interplay of environment, behavior, and molecular biology combined to reverse a declining trend in tuberculosis. To combat the new epidemic of tuberculosis, the public health infrastructure was rebuilt. Multidrug regimens (biologic efforts) and directly observing therapy to ensure compliance (behavioral efforts) were initiated and the rate of tuberculosis began to decline again.
INDIRECT EVIDENCE FOR CAUSE
In clinical medicine, it is not possible to prove causal relationships beyond any doubt, as one might a mathematic formula. What is possible is to increase one’s conviction in a cause-and-effect relationship by means of empiric evidence to the point where, as a practical matter, cause has been established. Conversely, evidence against a cause can accumulate to the point where a cause-and-effect relationship becomes implausible.
A postulated cause-and-effect relationship should be examined in as many different ways as possible. In the remainder of this chapter, we discuss some commonly used approaches.
Examining Individual Studies
One approach to evidence for cause-and-effect has been discussed throughout this book: in-depth analysis of the studies themselves. When an association has been observed, a causal relationship is established to the extent that the association cannot be accounted for by bias and chance. Figure 12.5 summarizes a familiar approach. One first looks for bias and how much it might have changed the result, and then whether the association is unlikely to be by chance. For observational studies, confounding is always a
possibility. Although confounding can be controlled in comprehensive, state-of-the science ways, it is almost never possible to rule it out entirely; therefore, confounding remains the enduring challenge to causal reasoning based on observational research.
possibility. Although confounding can be controlled in comprehensive, state-of-the science ways, it is almost never possible to rule it out entirely; therefore, confounding remains the enduring challenge to causal reasoning based on observational research.
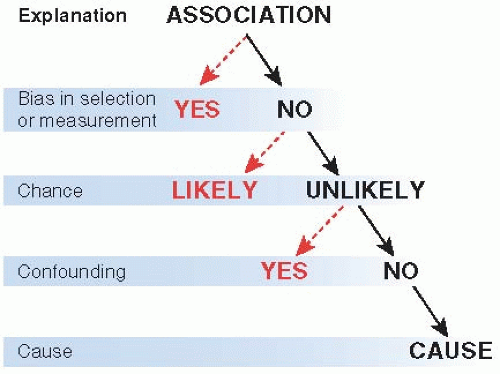 Figure 12.5 ▪ Association and cause. Bias, chance, and confounding should be excluded before concluding that a causal association is likely. |
Randomized trials can deal definitively with confounding, but they are not possible for studies of risk (i.e., causes) per se. For example, it is unethical (and would be unsuccessful) to randomize non-smokers to cigarette smoking to study whether smoking causes lung cancer. However, randomized controlled trials can contribute to causal inference in two situations. One is when the trial is to treat a possible cause, such as elevated cholesterol or blood pressure, and the outcome is prevented. Another is when a trial is done for another purpose and the intervention causes unanticipated harms. For example, the fact that there were an excess of cardiovascular events in randomized trials of the cyclooxygenase-2 inhibitor rofecoxib, which had been given for other reasons (e.g., pain relief), is evidence that this drug may be a cause of cardiovascular events.
Hierarchy of Research Designs
The various research designs can be placed in a hierarchy of scientific strength for the purpose of establishing cause (Table 12.2). At the top of the hierarchy are systematic reviews of randomized controlled trials because they can deal definitively with confounding. Randomized trials are followed by observational studies, with little distinction between cohort and casecontrol studies in an era when case-control analyses are nested in cohorts sampled from defined populations. Lower still are uncontrolled studies, biologic reasoning, and personal experience. Of course, this order is only a rough guide to strength of evidence. The manner in which an individual study is performed can go a long way toward increasing or decreasing its validity, regardless of the type of design used. A bad randomized controlled trial contributes less to our understanding of cause than an exemplary cohort study.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree