BACKGROUND
The impact of pregestational diabetes and gestational diabetes upon the mother during pregnancy is covered in other chapters. Adverse consequences for the fetus and the neonate arise either from the directly harmful metabolic environment, or the obstetric interventions required when maternal control is poor, or from inappropriate “routine” practices. Optimizing diabetic control minimizes risks to the mother and fetus, and reduces the risk of the postnatal complications described below. Whilst in many cases this is achieved and a healthy mother and baby result, it is important to be aware of the complications that can occur.
As the population of women with Type 2 diabetes becomes younger, particularly in some ethnic groups, the proportion of women with pregnancies complicated by pregestational Type 2 diabetes has risen to approximately one-third of pregnancies complicated by diabetes.1,2 These women have perinatal mortality rates and rates of fetal macrosomia that are no different from those with Type 1 diabetes.1 Finally, the fetus and neonate of the mother who develops gestational diabetes are at risk of some of the same adverse consequences if the gestational diabetes is not recognized and well managed.3
CARE OF THE HEALTHY INFANT AFTER PREGNANCY COMPLICATED BY DIABETES
For many women, especially those who access prenatal counseling and enhanced diabetes care and then continue to have good control during pregnancy, fetal and neonatal complications related to diabetes in pregnancy are unlikely. It is important to recognize that a baby at very low risk of complications should be managed according to normal standards for the healthy newborn baby.4,5 In particular, it is important to avoid unnecessary separation of mother and baby, and to facilitate successful breastfeeding if this is the mother’ s chosen method of feeding. Failure to follow these principles and the resulting iatrogenic complications are also covered below.
NEONATAL COMPLICATIONS – ETIOLOGY AND MANAGEMENT
Despite the aspiration that improved maternal diabetes care will minimize perinatal morbidity and mortality, recent data suggest that despite some improvements over time, insufficient progress has been made.1,2,6–11 Some neonatal complications arise from the effects of being born preterm or by cesarean section, and are not specific to diabetes, while others are secondary to intrauterine or intrapartum hypoxia–ischemia or the abnormal diabetic metabolic environment that the fetus may be exposed to during pregnancy. Finally, some neonatal problems are iatrogenic ( Tables 22.1 and 22.2).
Table 22.1 Neonatal complications after diabetes in pregnancy.
| Directly related to diabetes in pregnancy |
| • Congenital anomalies |
| • Intrauterine growth restriction |
| • Intrapartum hypoxia-ischemia |
| • Macrosomia, obstructed labor, birth injury |
| • Neonatal death |
| • Polycythemia/jaundice |
| • Hypoketonemic hypoglycemia |
| • Hypocalcemia, hypomagnesemia |
| • Hypertrophic cardiomyopathy |
| Complications of necessary, or unnecessary, obstetric interventions |
| • Complications of preterm delivery |
| • Complications of cesarean section–respiratory distress, impact on breastfeeding |
| Iatrogenic |
| • Inappropriate separation of mother and baby |
| • Inappropriate formula supplementation–impact on breastfeeding |
Table 22.2 Neonatal outcomes in the UK.1
Perinatal mortality
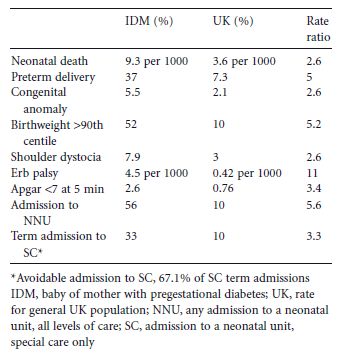
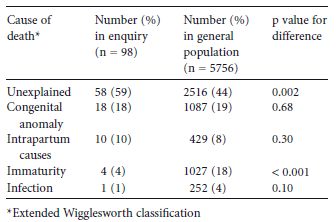
Data from the UK for 2002–2003 indicate that the overall UK perinatal mortality rate (stillbirths and first-week neonatal deaths) with pregestational diabetes was 31.8 per 1000 compared with a national rate of 8.5 per 1000 (RR 3.8, 95% CI 3.0–4.7).1 This was similar to the rates in cohort studies from The Netherlands (1999–2001),6 Scotland (1998–1999),10 English Northern region (1996–2004),2 and North West England (1990–1994).9 Higher rates of 37 per 1000 and 48 per 1000, respectively, were reported in older cohort studies from Scotland (1979–1995)11 and the Northern region (1994).8 For the cohort of babies from the Confidential Enquiry into Maternal and Child Health (CEMACH) undergoing a more detailed enquiry, the most common causes of death were related to congenital abnormality and intrapartum complications (Table 22.3).7
Severe fetal compromise resulting in intrauterine loss is covered in chapter 12. The stillbirth rate for women with Type 1 and Type 2 diabetes in the UK 2002–2003 cohort was 26.8 per 1000, compared with a national rate of 5.7 per 1000 (RR 4.7, 95% CI 3.7–6.0).1 Similar rates have been reported in other UK and European studies.6,9–11
These are pregnancies at the extreme of the spectrum for adverse sequelae of diabetes in pregnancy; it follows that fetuses less severely affected will survive, but carry a burden of neonatal compromise. Indeed, in the UK CEMACH cohort, the neonatal death rate was 9.3 per 1000 for babies born to mothers with diabetes, compared with a national rate of 3.6 per 1000 (RR 2.6, 95% CI 1.7–3.9).1 Neonatal mortality rates were very similar to this in the Dutch and Scottish cohort studies,6,11 but were higher in the older studies from the UK Northern Region and North West England.8,9
Table 22.3 Causes of perinatal mortality in the UK.1
Preterm delivery
In the UK CEMACH cohort (2002–2003), the rate of preterm delivery (< 37 weeks of gestation) for babies born to mothers with pregestational diabetes was 35.8% compared to a rate of 7.4% in the general population.1 The Netherlands cohort study has provided similar data.6 The causes of preterm delivery are covered in chapter 20.
As with other maternal conditions that affect pregnancy, there is always a balance between continuing a pregnancy until term and reducing the time that both fetus and mother are exposed to a harmful environment. However, for women in the UK cohort, 19% had preterm delivery that was not spontaneous or explained by maternal or fetal compromise and thus could have been avoided.1 This would have prevented some 235 admissions to neonatal care over the study period.
If preterm delivery is planned, this must be in a unit that can provide neonatal intensive care, which may require transfer of the mother to an appropriate unit, preferably within a perinatal network system as operates in the UK.
Now that it is widely recognized that mothers with diabetes should receive steroid injections if preterm delivery is anticipated, babies of diabetic mothers do not in general have worse respiratory distress than other babies of equivalent gestation. In the UK cohort, 70% of women who delivered live babies between 24 and 34 weeks of gestation received prophylactic antenatal steroids.1 However, there have been concerns regarding potential worsening of maternal glycemic control and resulting perinatal and maternal morbidity if steroid therapy is given. In the UK cohort, for five of the 68 women who were not given steroids, the reason cited was “health professionals concerned about effect of steroids on maternal glycemic control”.7 The rationale for giving steroid therapy and subsequent maternal management are discussed elsewhere (chapter 21).
If a baby is born preterm, there is no evidence that the usual complications of prematurity are more severe than for a baby born at a similar gestational age to a mother who does not have diabetes. Preterm babies of diabetic mothers should be managed according to standard protocols. In particular, mothers should be encouraged to express and store breast milk. Additional problems specific to the baby of a diabetic mother may be present and need additional management (see below).
Effects of delivery by cesarean section
In the 2002–2003 UK cohort, the cesarean section rate for women who have diabetes was 67%1 and in The Netherlands study 44.3%,6 compared with the overall UK national rate of 22%. In the UK study, 9% of cesarean sections were not explained by maternal or fetal compromise and 4% were “routine for diabetes” or “maternal request”.1 As stated above, a number of these “routine” cesarean sections were at a preterm gestation. Even in pregnancies complicated by gestational diabetes (rather than pregestational diabetes), there appears to be a higher rate of cesarean section (see chapter 20).
Whilst the baby may be protected from hypoxic–ischemic brain injury by avoiding labor and vaginal delivery, the potential adverse impacts on the baby of unnecessary cesarean section are two-fold: delayed and disrupted breastfeeding and respiratory morbidity (transient tachypnea of the newborn or surfactant deficiency).12,13 These in turn frequently result in avoidable admission to a neonatal unit and separation of mother and baby.
Effects of antenatal and intrapartum hypoxia–ischemia
Hypoxia–ischemia is the combined pathology of impaired oxygenation of the blood and reduced perfusion (secondary to the effect of hypoxia on cardiac function). This is potentially damaging to all organ systems, and particularly the brain. The mechanisms by which intrauterine loss and neonatal complications occur secondary to hypoxia–ischemia are not fully understood (see chapters 3 and 12). However, it is likely that macrosomia and obstructed labor may contribute to intrapartum hypoxia–ischemia and increase the risk of neonatal complications.
In the UK enquiry, 10% of perinatal deaths were related to intrapartum causes.7 These represent the end of a spectrum; many babies affected by intrapartum hypoxia–ischemia will be born alive and require expert resuscitation. This is one of the reasons why delivery of babies of diabetic mothers must occur in units where advanced neonatal life support is available. If a neonate has unexpected and severe complications of hypoxia–ischemia, he/she will require transfer to a neonatal unit which provides intensive care, if this is not available in the hospital of birth. As total body cooling becomes an established treatment for hypoxic–ischemic encephalopathy, then time is of the essence in commencement of this treatment at a specialist center.14
Relative cellular hypoxia causes increased erythropoietin secretion and in turn increased fetal red cell production.15 The resulting neonatal polycythemia may then cause excessive neonatal jaundice (as the red cell burden is lyzed) and occasionally hyperviscosity syndrome. Renal vein thrombosis or thrombosis in other vessels is rare, but occurs more frequently in babies whose mothers have diabetes compared to those whose mothers do not.
Clinicians caring for these babies must be alert to these complications and test for them if there are abnormal clinical signs, such as irritability, lethargy, and poor feeding. The effects of polycythemia and hypoglycemia may be additive in terms of reduction of glucose delivery to the brain, and polycythemia associated with clinical signs, such as irritability or lethargy, must be treated with partial exchange transfusion, according to standard neonatal guidance.
Congenital anomalies
It has long been recognized that there is a higher incidence of congenital anomalies in pregnancies complicated by diabetes than in the general population.16 The most recent UK data demonstrated that 4–6% of fetuses of diabetic mothers had one or more major congenital anomalies.1,10 The reported incidence was higher in The Netherlands and in the older UK cohort studies from the North East and North West of England.6,8,9
The most common anomalies are congenital heart disease (the incidence in the CEMACH cohort 1.7% was three times that in the general population) and anomalies of limb, musculoskeletal system or connective tissue (incidence 0.7%).1 Neural tube defects, although numerically rare, are 3.4 times more common than in the general population.1 The possible etiologies of these anomalies and strategies for their prevention are covered in chapters 8 and 14.
The most important predelivery issues for the obstetrician and neonatologist are to ensure that there has been adequate counseling of parents, involving the specialist team who will care for the baby postnatally, and to ensure that delivery takes place at an appropriate center (dependent on the nature of the anomaly) to enable early access to specialist care. Routine postnatal echocardiography to screen for congenital heart anomalies is not indicated, unless an abnormality has been suspected on antenatal scanning or the baby presents with clinical signs of congenital heart disease.5
Macrosomia – obstructed labor, birth injury, and organomegaly
Macrosomia and large for gestational age are not interchangeable terms. Macrosomia describes a baby who is heavier than his/her genetically deteremined birthweight, has the clinical appearance of a baby who has had somatic growth in excess over head growth, and may be present in a baby of “normal” birthweight. Macrosomia and organomegaly attributed to fetal hyperinsulinemia are well-recognized characteristics of pregnancies complcated by diabetes, but evidence is inconsistent regarding the potential impact on these morbidities of improved diabetic control and duration of diabetes.1,3,11,17 The rate of macrosomia (birthweight above 90th centile) was 52% in the recent UK cohort.1
The clinical significance of macrosomia pertains to the the risk of complications presented by delivery of a large infant, such as shoulder dystocia, obstructed labor, perinatal hypoxia–ischemia, and birth injury (e.g. brachial plexus injury and fractured clavicle or humerus). Recent UK cohort data provide rates for some of these: shoulder dystocia 7.9% (over twice the rate in the general population), Erb palsy 4.5 per 1000 births (10 times the rate in the general population), and fractures (usually of the clavicle and humerus) 7 per 1000 births.1 In the context of the high rate of preterm delivery and cesarean section in this cohort, the complication rate is likely to be even higher with more normal deliveries at term.
Management of these complications is covered in standard neonatal texts. Some, such as fractures, cause no long-term morbidity, but significant long-term neurodevelopmental morbidity may be associated with hypoxia – ischemia secondary to obstructed labor and Erb palsy.
Finally, parents and health professionals must be prepared for “catch down” in postnatal growth of macrosomic babies, especially when breastfed. This is a normal and healthy adaptation, and provided the baby appears to be feeding well and is healthy, there should be no concern if there is an initial period of slow weight gain such that weight trajectory crosses down the centile lines. Rather, to over feed the baby and have him/her remain overweight has long-term health consequences, e.g. later risk of cardiovascular disease and diabetes.9 This is a further reason to promote and support breastfeeding, which protects against long-term metabolic disturbances.18,19
Hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy, characterized by hypertrophied septal muscle which obstructs the left ventricular outflow tract, may be sufficiently severe to cause fetal or neonatal death.15 In less severe cases, the presentation is usually within the first weeks of postnatal life with cardiorespiratory distress and congestive heart failure. The majority of infants need supportive care only, as resolution of the signs can be expected in 2–4 weeks. The septal hypertrophy regresses within 2–12 months. Routine postnatal echocardiography is not required unless there are clinical signs.5
Intrauterine growth restriction
Intrauterine fetal growth restriction, often associated with severe diabetic vasculopathy, may lead to further problems after birth. The small for gestational age infant of the diabetic mother appears to be at even greater risk of adverse outcome, especially neurodevelopmental sequelae.20 Often this is compounded by a requirement for preterm delivery. Delivery must be planned at an appropriate unit as specialist neonatal care is likely to be required.
Impaired postnatal metabolic adaptation
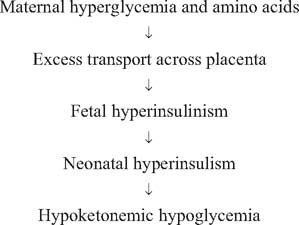
With the cessation of placental nutrition at birth, the healthy newborn baby undergoes metabolic adaptation to ensure energy provision to vital organs and subsequently to sustain growth and further development. The key fuels are glucose and ketone bodies (the latter being the product of beta oxidation of fatty acids). The infant of the diabetic mother is at risk of transient hyperinsulinism, which in turn causes a high rate of glucose uptake and conversion to fat, reduced hepatic glucose production, and reduced lipolysis and thus reduced ketone body production15,21 (Fig. 22.1). At the extreme end of the spectrum, this will result in hypoketonemic hypoglycemia with markedly reduced fuel availability for the brain and other vital organs.
The ultimate concern is that of brain injury and long-term neurodevelopmental sequelae. Reviews of a number of published studies have suggested an association between the occurrence of neonatal hypoglycemia and adverse neurodevelopmental outcome, but none has been able to exclude other potentially confounding complications of maternal diabetes, which may also influence outcome.15,21 Whilst it is clear that untreated hypoglycemia that is sufficiently severe and prolonged to cause clinical signs may cause brain injury, there is no evidence that brain injury occurs in the absence of clinical signs (“asymptomatic hypoglycemia”). Clinical signs suggestive of (but not specific to) hypoglycemia are:
- Abnormal tone
- Abnormal level of consciousness
- Poor oral feeding
- Fits which may be atypical, e.g. presenting as apnea.
Fig. 22.1 Impaired neonatal metabolic adaptation.