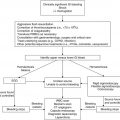
Clinical scenario
Recommendations
Level of evidencea
SVCS/SMS
Elevate head of the bed
1C
Place all IV lines in lower extremities
2C
Consider use of loop diuretics
2C
Remove indwelling catheter if associated with thrombosis
1C
Avoid sedation with general anesthesia if possible
1B
Consider use of pre-biopsy steroids to shrink tumor
2C
Consider pre-biopsy radiation therapy to shrink tumor
2C
Malignancy-associated pericardial effusions
Close observation only if patient is asymptomatic
1C
Emergent pericardiocentesis under echocardiographic guidance if patient is symptomatic
1C
Consider pigtail drainage catheter for prolonged drainage
2C
Surgical management if not controlled by above methods
1C
Volume resuscitation to elevate intracardiac pressures
2C
Avoid mechanical ventilation with positive airway pressure
2C
Malignancy-associated pleural effusions
Close observation only if patient is asymptomatic
1B
Thoracentesis if patient is symptomatic (send fluid for evaluation, including cytology)
1B
Consider indwelling catheter for prolonged drainage
2C
Intrapleural fibrinolysis for loculated effusion
1A
VATS for early organizing empyema
1C
Thoracotomy with decortication for advanced organizing empyema
1C
Consider pleurodesis for refractory and recurrent effusion
2C
Hypertensive emergencies
Immediate intravenous therapy for severe hypertension
1C
Rapid bolus of phentolamine
Continuous infusion of phentolamine or sodium nitroprusside
Avoid β-blocking agents initially
Oral therapy for less severe hypertension
1C
α-blocking agent (i.e., doxazosin)
Calcium channel blocker
Consider addition of β-blocker after α-adrenoreceptor blockade
Prevention of hypertensive crisis if marked catecholamine release anticipated
1C
α-adrenoreceptor blockade with phenoxybenzamine
Subsequent addition of β-blockade to prevent reflex tachycardia
4.2 Superior Vena Cava and Superior Mediastinal Syndromes
Superior vena cava syndrome (SVCS) occurs when the superior vena cava is obstructed, thereby restricting blood return to the heart. Superior mediastinal syndrome (SMS) is the term used when SVCS coexists with obstruction of the trachea. The terms are sometimes used interchangeably in children, in whom mediastinal pathology frequently involves compression of both the SVC and the trachea. Malignancies are the most common cause of this condition. The most common pediatric malignancy associated with SVCS is lymphoma, but it can also be seen in other solid tumors such as germ cell tumors, neuroblastoma, Ewing sarcoma, and soft tissue sarcoma, as well as leukemia (Ingram et al. 1990; Halfdanarson et al. 2006).
4.2.1 Pathophysiology
The superior vena cava carries blood from the head, arms and upper torso to the heart. This thin-walled vessel is easily compressed by tumors or other pathology in the mediastinum leading to impedance in venous return. If the occlusion occurs gradually, collaterals may form thereby mitigating the symptoms. Airway compromise results from both direct compression of the tracheobronchial tree and edema of these airways due to venous engorgement.
4.2.2 Clinical Presentation and Diagnosis
4.2.2.1 History and Physical Exam
SVCS/SMS should be suspected in a patient with engorgement of the veins in the head, face and neck. The most common presenting signs and symptoms are facial swelling and plethora, distended neck and chest wall veins, and upper extremity edema. Respiratory symptoms include dyspnea and cough. Less frequently seen are central nervous system (CNS) symptoms caused by impeded venous return and subsequent cerebral edema. These can include headache, dizziness, confusion, syncope and even obtundation (Wilson et al. 2007a). The onset of symptoms is usually insidious but may occur rapidly especially when the underlying cause is a rapidly growing tumor.
4.2.2.2 Imaging Studies
The diagnosis of SVCS/SMS is often made on the basis of clinical signs and symptoms but imaging studies are helpful in determining the underlying etiology. A chest radiograph is easy to obtain and can confirm the presence of a mediastinal mass. A computed tomography (CT) scan of the chest is typically the most useful imaging study and should be obtained after the administration of intravenous (IV) contrast to best evaluate the SVC. CT can provide information regarding the exact size and location of the mass (which can provide clues regarding its etiology), as well as infiltration into surrounding structures and vascularity. It is important to keep in mind that patients with SMS are at high risk for adverse cardiorespiratory events if sedated. Ultrasonography can be performed when sedation is not possible or if the patient cannot lie supine. Echocardiography may be required if there is suspicion of infiltration by the mass into the pericardial cavity or pericardial effusion, based on physical exam or CT scan findings.
4.2.2.3 Other Studies
Tissue is required to make a definitive diagnosis and should be obtained by the least invasive procedure possible to reduce the possibility of an adverse cardiorespiratory event. If there is an associated pleural or pericardial effusion, thoracentesis or pericardiocentesis respectively may be not only therapeutic but also diagnostic in approximately 50 % of cases (Rice et al. 2006). An enlarged palpable lymph node may be more easily biopsied than a mediastinal mass. Mediastinoscopy is more invasive but has the highest diagnostic yield, and some studies have reported low complication rates even in the presence of SMS (Dosios et al. 2005).
4.2.3 Treatment
SVCS/SMS is not typically considered to be a true medical emergency unless airway compromise or neurologic symptoms are present (Yellin et al. 1990). However, tumors with rapid growth can lead to a rapid progression of symptoms. Management consists of both relief of the obstructive symptoms and treatment of the underlying malignancy (Table 4.1). Most data regarding management of SVCS come from case series as there have not been many randomized controlled trials; such studies comparing management options for patients with SVCS/SMS have had difficulty accruing patients (Wilson et al. 2007b). Elevation of the head of the bed can assist in venous drainage and decreasing edema (Cheng 2009). All IV lines should be placed in the lower extremities, so as to prevent further raised hydrostatic pressure in the SVC. Loop diuretics are sometimes used, although it is unclear whether these agents have a significant effect on the rate of clinical improvement (Schraufnagel et al. 1981). In patients with SVC obstruction resulting from intravascular thrombosis associated with an indwelling catheter, removal of the catheter should be strongly considered.
Sedation should be avoided as much as possible, as sedative medications result in reduced respiratory drive and relaxation of bronchial muscles. When a biopsy is required, obtaining tissue under local anesthesia should be the goal. The indicators of risk for general anesthesia in such patients are controversial. Large retrospective reviews have not identified any single clinical sign or test that can accurately predict which patients with a mediastinal mass will experience complications under general anesthesia (Hack et al. 2008). Patients with stridor, orthopnea, wheezing, SVC obstruction, CNS symptoms, pericardial effusion, tracheal or bronchial compression >50–70 %, pulmonary artery outflow obstruction, or peak expiratory flow rate (PEFR) of <50 % are considered to be at higher anesthetic risk (Hack et al. 2008). Others have suggested that PEFR and tracheal cross-sectional area seem to be the most reliable criteria in identifying children at greatest risk for anesthetic complications, and that if both the PEFR and the tracheal cross-sectional area are >50 % of predicted values general anesthesia can be administered safely (Ricketts 2001). If general anesthesia is considered necessary, spontaneous ventilation should be maintained if possible.
As lymphoma and leukemia are among the most common causes of a mediastinal mass and resultant SVCS in children, many centers recommend delaying diagnostic biopsy in symptomatic patients for 24–48 h while emergent corticosteroids or radiation are given in an effort to shrink the tumor size. However, there is concern that such pretreatment can distort cellular morphology and thereby adversely affect the ability to make a histologic diagnosis (Ferrari and Bedford 1990). Retrospective reviews have found that pre-biopsy steroid or radiation treatment caused delay or failure of definitive diagnosis or staging in a minority of children; fortunately many of these patients who were empirically treated did well and have remained disease-free at last follow-up (Loeffler et al. 1986; Borenstein et al. 2000). In an effort to preserve tumor histology for diagnostic purposes, radiation oncologists should attempt to spare radiation to a limited portion of the tumor, with the later goal of obtaining untreated biopsy tissue from that region. This limited “postage stamp” approach to the radiation field is also used to safely treat various types of tumors (Slotman et al. 1996; Hayakawa et al. 1999). Alternate tissue sources from which to make the diagnosis should be considered when possible, including pleural or pericardial effusions and enlarged palpable lymph nodes (see Sect. 4.2.2.3). Ultimately, the decision to treat such a patient prior to obtaining tissue for biopsy should depend on the patient’s clinical status and severity of cardiorespiratory symptoms.
4.3 Pericardial Effusion and Cardiac Tamponade
Pericardial effusions are common in adult malignancy, with some series reporting this condition in up to one-third of patients with cancer (Wilkes et al. 1995; McCurdy and Shanholtz 2012). Such effusion can be caused by direct tumor invasion into the pericardium or, rarely, by metastases from other locations. While frequently asymptomatic, severe cases can lead to compression of the heart chambers and cardiac tamponade (Medary et al. 1996).
4.3.1 Pathophysiology
The pericardium is composed of a serous layer of mesothelial cells adherent to the surface of the heart and a fibrous parietal layer formed by the pericardium and reflecting back on itself. The space between these two layers contains up to 50 mL of fluid which serves as a lubricant. Excess fluid can accumulate in this space without affecting the pericardial pressure until it reaches the volume that begins to distend the pericardium, termed the pericardial reserve volume. Once this reserve volume is reached, pressure begins to rise sharply due to the relative inextensibility of the pericardium. The heart is then forced to compete with the increased pericardial contents for the fixed intrapericardial volume which in turn leads to an impaired filling of the cardiac chambers and hemodynamic compromise. This compression of the heart due to the pericardial accumulation of fluid is termed cardiac tamponade and can be life threatening (Spodick 2003).
4.3.2 Clinical Presentation and Diagnosis
4.3.2.1 History and Physical Exam
Small pericardial effusions are frequently asymptomatic (Maher et al. 1996). The amount of pericardial fluid that causes tamponade is related to the rate of fluid accumulation. Rapidly accumulating effusions can cause symptoms with as little as 200 mL of fluid, whereas if fluid accumulates over weeks to months, the pericardial tissue can stretch and may hold >2 L or more before tamponade develops (Karam et al. 2001). In the case of malignancy, the onset is more often insidious.
The most common presenting symptom is exertional dyspnea (Wilkes et al. 1995). Other symptoms include cough, chest pain, dysphagia, hoarseness and hiccups (Karam et al. 2001). The most common sign is pulsus paradoxus, which is defined as a decrease in systolic blood pressure of more than 10 mmHg during inspiration. Tachycardia is frequently seen. The classic description of cardiac tamponade is Beck’s triad: hypotension, increased jugular venous pressure and quiet heart sounds. However, this is seen mostly in rapidly forming effusions and acute tamponade, and only infrequently in patients with chronic pericardial effusion (Tseng et al. 1999).
4.3.2.2 Imaging and Other Studies
The presence of a pericardial effusion can be suspected based on chest radiograph findings which classically show an enlarged cardiac silhouette. The classic appearance of a “water bottle heart” that is globular in appearance is a sensitive but nonspecific finding (Fig. 4.1). An electrocardiogram may reveal low-voltage waveforms and less frequently electrical alternans, a condition where consecutive QRS complexes alternate in height between beats (Karam et al. 2001). Echocardiography, however, has become the preferred diagnostic test for assessing pericardial effusion and cardiac tamponade. It should be ordered when there is a significant pericardial effusion suspected, as it can not only define the size and location of an effusion but also assess the hemodynamic significance and help guide pericardiocentesis (Tseng et al. 1999).
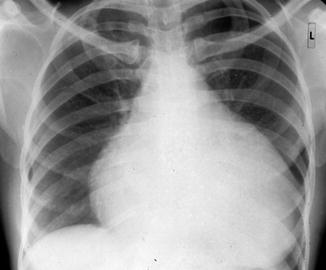

Fig. 4.1
Enlarged (“water bottle”) silhouette of the heart on chest radiograph in a patient with pericardial effusion
4.3.3 Treatment
The timing and type of treatment for pericardial effusion depend on the severity of symptoms (Table 4.1). If the effusion is asymptomatic, continued close observation for hemodynamic complications is required but the effusion generally resolves with treatment of the underlying malignancy (Bashir et al. 2007). In the case of tamponade or if the pericardial effusion is otherwise hemodynamically significant, the fluid should be drained emergently. Pericardiocentesis under echocardiographic guidance is the therapy of choice and has been shown to be a safe and effective treatment for pediatric oncology patients with symptomatic pericardial effusion or tamponade (Medary et al. 1996). In addition to relieving symptoms related to the effusion, this procedure can be used to determine the etiology of a malignant effusion. As the recurrence rate of pericardial effusions after initial drainage can be as high as 50 %, consideration should be given to introducing an indwelling pigtail drainage catheter at the time of pericardiocentesis to allow for prolonged drainage (Tsang et al. 1999). Surgical management of malignant pericardial effusion should be reserved for the rare case that cannot be controlled by this method (Maher et al. 1996). In the case of acute cardiac tamponade, the patient should receive volume resuscitation to elevate intracardiac pressures to greater than pericardial pressures. Mechanical ventilation with positive airway pressure should be avoided in these patients because it can further decrease cardiac output (Spodick 2003).
4.4 Pleural Effusion
A pleural effusion is a collection of fluid that accumulates between the visceral and parietal pleurae. Excessive amounts of such fluid can impair breathing by limiting lung expansion during ventilation. In the pediatric population, the most common cause of a pleural effusion is an underlying pneumonia, followed by congenital heart disease and less commonly malignancy (Beers and Abramo 2007). Severe cases can lead to significant respiratory compromise and may require emergent management.
4.4.1 Pathophysiology
The pleural space is bordered by the parietal pleura which covers the inner surface of the thoracic cavity and the visceral pleura which covers the lung surfaces. Like the pericardial space, there is normally a small amount of lubricating fluid between these two layers, but this fluid can increase secondary to underlying pathology. Pleural effusions are classified as either transudates or exudates. Exudates are typically of infectious etiology and result from inflammation on the pleural surface. Transudates, on the other hand, are generally of noninfectious etiology and result from an imbalance between the rate of pleural fluid formation and its reabsorption as the distribution of hydrostatic and oncotic pressure across the pleura is altered.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree