Fig. 3.1
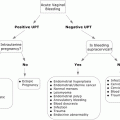
A 36-year-old woman with metastatic thyroid cancer and no prior history of arrhythmia presented to the emergency room with a chief complaint of palpitations. Cardiac monitor tracings demonstrated a baseline sinus rhythm (a) with paroxysms of symptomatic supraventricular tachycardia, atrial fibrillation (b), and ventricular tachycardia. Echocardiograms demonstrated evidence of multiple metastatic lesions involving the interventricular septum (c) and the left ventricular apex (d). Myocardial tumor infiltration was felt to be responsible for the arrhythmia
Table 3.1
Potential causes of cardiac arrhythmias in patients with malignancy
Malignancy-related |
Pericardial infiltration |
Myocardial metastasis |
Carcinoid valvular heart disease |
Carotid compression |
Cancer therapy-related |
Surgery/radiation therapy involving the neck |
Baroreflex failure |
Chemotherapy-induced cardiomyopathy |
Chemotherapy drug-related |
Bradyarrhythmia |
Sinus bradycardia (thalidomide, paclitaxel, high-dose steroids, antiemetics) |
AV block (paclitaxel) |
Tachyarrhythmia |
Sinus tachycardia |
Atrial fibrillation (vemurafenib) |
Atrial tachycardia (ifosfamide) |
Ventricular tachycardia (interleukin-2, methotrexate) |
QT prolongation/torsades de pointes (arsenic trioxide, vorinostat, nilotinib, lapatinib, dasatinib) |
Diagnosis and Management
When managing cancer patients with suspected acute arrhythmia, emergency care providers should be vigilant and administer treatment to the whole patient and not just for the rhythm disturbance. These patients have complex associated comorbidities, and a rapid heart rate or rhythm irregularities can be simply signs of much more complicated and severe acute illness (e.g., atrial tachycardia or atrial fibrillation in the setting of acute pulmonary embolism, polymorphic ventricular tachycardia triggered by severe metabolic derangements and electrolytes imbalance while taking a QT interval-prolonging agent) (Fig. 3.2).
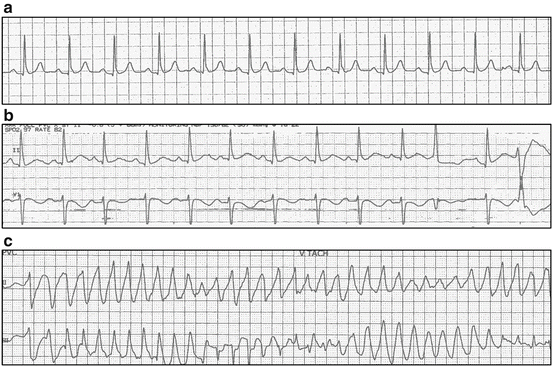

Fig. 3.2
A 68-year-old woman was admitted for recurrent syncope with documented severe hypokalemia (K: 2.8) and hypomagnesemia (Mg: 1.5) while taking voriconazole and ondansetron following chemotherapy (cytarabine and idarubicin) for leukemia. Unlike her previous normal baseline QT interval (a), the admission electrocardiogram tracings demonstrated a prolonged QT interval (b). She subsequently had documented symptomatic polymorphic tachycardia (c) episodes while being monitored
In the absence of clinical data suggesting otherwise, acute management of cardiac arrhythmia in cancer patients should follow the well-established standard of care guidelines (Blomstrom-Lundqvist et al. 2003), although it can differ slightly from that in patients without malignancies. The difference is mainly related to the choice of antiarrhythmic drugs and atrioventricular (AV)-blocking agents and also the timing and safety of anticoagulation. The selection of these drugs should take into consideration the possibility of drug-drug interactions. For example, diltiazem (Cardizem) and verapamil are potent cytochrome P450 inhibitors that can alter the pharmacokinetics of many chemotherapeutic agents. The QT interval prolongation observed with the use of many cancer therapies can be potentiated by several classes of antiarrhythmic drugs. Also, whether to use short-term or long-term anticoagulation for atrial fibrillation or flutter should be determined carefully in each case, as many patients face increased risk of bleeding in the setting of thrombocytopenia secondary to malignancy or its therapy.
Acute management of arrhythmia in the emergency room starts with the identification of any alarming symptoms resulting from underperfusion of vital organs. These include hypotension, angina, myocardial infarction, heart failure, altered mental status, and shock. For patients presenting with any of these symptoms, immediate electrical cardioversion is indicated if tachycardia is present, and cardiac pacing is indicated if bradycardia is the cause. For patients with relatively stable hemodynamics, the focus should be establishing a specific diagnosis and mechanism of arrhythmia using 12-lead electrocardiography and the clinical response to vagal maneuvers or drugs. Arrhythmias then can be classified as bradycardia or tachycardia.
Bradycardia
Bradycardia is defined as a heart rate under 60 beats per minute. A physiologic low heart rate must be distinguished from bradycardia associated with a serious cardiac pathology (e.g., sinus syndrome, heart block).
Etiologies and Mechanisms
A careful review of all medications must be performed to eliminate pharmacotherapy that could lower the heart rate. Certain chemotherapeutic agents have been linked with bradycardia (Table 3.1). The most common of these include paclitaxel and thalidomide. In early phase 1 clinical trials, paclitaxel caused serious hypersensitivity reactions. Thalidomide has been associated with bradycardia at a lower frequency, but the pathophysiology is unclear (Yeh and Bickford 2009).
A less common but equally important cause of bradycardia is baroreflex failure. It is typically characterized by heart rate and blood pressure volatility. Baroreflex failure can arise from abnormalities in the vascular baroreceptors, glossopharyngeal or vagal nerves, or brain stem. This is most often seen in cancer patients who undergo extensive head and neck surgery or receive radiotherapy, which can cause inflammation and scarring of the neck vessels.
Treatment
After identifying and removing any potentially offending agents that can exacerbate bradycardia, treatment must be individualized depending on the symptoms. Careful clinical judgment must be used in determining the cause of the bradycardia and deciding whether it is reversible. For severely symptomatic patients, urgent medical therapy with atropine or an intravenous (IV) inotrope, such as dopamine and epinephrine, may be used. In emergency situations, transcutaneous or transvenous pacemaker therapy may be required to maintain hemodynamic support. Long-term support with permanent pacing will depend on the severity of the symptoms related to the bradycardia and whether it is reversible.
Tachycardia
A clinically useful and practical approach to treating tachycardia permits the classification of it into four categories: irregular, regular narrow QRS complex, wide QRS complex, and polymorphic ventricular tachycardia. A clinically practical stepwise approach to diagnosis and classification of tachycardia is summarized in Fig. 3.3.
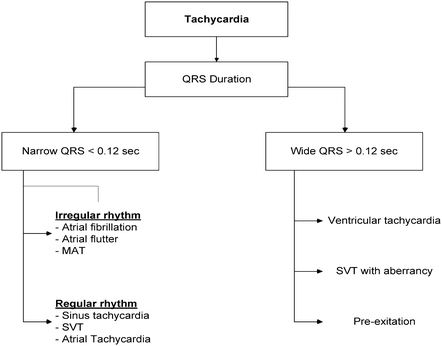

Fig. 3.3
Electrocardiogram-based approach to diagnosis and classification of tachyarrhythmia. MAT multifocal atrial tachycardia
Narrow QRS Complex Tachycardia
Narrow QRS complex tachycardia is almost always supraventricular in origin (exceptions are rare) and indicates that electrical conduction occurs through the AV node. Patients with narrow QRS complex tachycardia typically present with palpitations, dizziness, and dyspnea and rarely present with syncope.
Treatment of Regular Narrow QRS Complex Tachycardia
If vagal maneuvers fail in the treatment of narrow QRS complex tachycardia, then treatment with adenosine, β-blockers , or nondihydropyridine calcium-channel antagonists (Cardizem or verapamil) should be tried. Caution is advised when using adenosine in patients with asthma or who have received theophylline (lack of effect) or dipyridamole (potentiates side effects). Atrial fibrillation can occasionally develop following adenosine injections for supraventricular tachycardia (SVT) . Immediate termination of the tachycardia suggests SVT (AV nodal re-entrant tachycardia or AV re-entrant tachycardia), whereas lack of response or transient, brief slowing of the heart rate is observed in the settings of sinus tachycardia, atrial tachycardia, atrial fibrillation, and atrial flutter.
Sinus and atria tachycardia are often secondary and triggered by other concomitant acute illnesses or procedures (e.g., infection, pneumonia, pulmonary embolism, surgery). Evaluation and treatment of the primary etiology and its precipitating causes are effective. In the occasional setting in which atrial tachycardia is persistent or poorly tolerated, pharmacologic intervention with adenosine, β-blockers, nondihydropyridine calcium-channel antagonists, or antiarrhythmic drugs (procainamide, flecainide, propafenone, amiodarone, and sotalol) can be helpful. These drugs have proven to be effective in conversion to sinus rhythm. For rate control, β-blockers, calcium-channel antagonists, and digoxin are effective in blocking the AV node (Blomstrom-Lundqvist et al. 2003).
In the setting of SVT , electrical cardioversion is recommended if tachycardia persists despite the use of vagal maneuvers, carotid massage, adenosine, β-blockers, or calcium-channel antagonists. Use of antiarrhythmic drugs for acute management of SVT is discouraged; they should only be used if the above-mentioned therapeutic measures are ineffective. Flecainide and propafenone are the most preferred antiarrhythmic drugs in the absence of underlying structural heart disease. Procainamide, amiodarone, sotalol, and disopyramide are also effective. In patients with known underlying left ventricular dysfunction, digoxin or amiodarone is preferred.
Treatment of Irregular Narrow QRS Complex Tachycardia
Acute management of atrial fibrillation and atrial flutter in the emergency room follows the general recommendations of urgent cardioversion for hemodynamically unstable patients and initial rate control for stable patients. Ventricular rate control can be achieved using AV-blocking agents like digoxin, β-blockers, and nondihydropyridine calcium-channel antagonists (Cardizem and verapamil). An amiodarone drip can also be considered for rate control in patients with marginal blood pressure or left ventricular dysfunction.
For the subgroup of patients with previously known and documented permanent atrial fibrillation or flutter, controlling the heart rate and reversing the cause of acute decompensation should suffice. For patients with no known prior history of arrhythmia, clinical decision-making regarding acute management is dependent on the arrhythmia. For patients with confirmed arrhythmia durations under 48 h, electrical or chemical cardioversion can be performed safely. Medications with proven efficacy for cardioversion include ibutilide, amiodarone, flecainide, propafenone, procainamide, sotalol, and disopyramide. Those with arrhythmia of unknown duration or suspected duration of more than 48 h have an increased risk of arterial embolization following cardioversion. These patients should receive adequate anticoagulation (e.g., warfarin) or oral direct thrombin inhibitors (e.g., dabigatran) for at least 3 weeks prior to cardioversion and then 4 weeks thereafter. An acceptable alternative is a transesophageal echocardiogram (TEE) in the absence of documented left atrial or left atrial appendage thrombus; cardioversion then can be performed safely, and anticoagulation can be initiated and continued for 4 weeks .
Multifocal atrial tachycardia is typically observed in the setting of lung disease and must be differentiated from atrial fibrillation, as both present as irregular arrhythmias. Antiarrhythmics and cardioversion are ineffective in the setting of multifocal atrial tachycardia. Rate control can be achieved with the use of nondihydropyridine calcium-channel antagonists. Anticoagulation is not indicated. Treating the underlying lung process (e.g., chronic obstructive pulmonary disease, hypoxia) can help control this arrhythmia.
Wide QRS Complex Tachycardia
Wide QRS complex tachyarrhythmias (QRS complex greater than 0.12 s) should be classified as one of two distinct entities: ventricular tachycardia or SVT with aberrant conduction. Patients with SVT tend to be hemodynamically stable and present with symptoms similar to those seen in patients with narrow QRS complex tachycardia. Electrocardiographic features may be helpful in distinguishing between SVT with aberrant conduction and ventricular tachycardia (Table 3.2).
Table 3.2
Clinical and ECG features favoring ventricular tachycardia
Clinical features | ECG features |
|---|---|
Prior myocardial ischemia/ischemic heart disease | Very wide QRS complex, QRS > 0.16 s |
Cardiomyopathy | Positive/negative concordance of precordial leads |
Structural heart disease | AV dissociation |
Family history of sudden death | Fusion beats |
Capture beats | |
Brugada sign (interval from beginning of R wave to deepest part of S > 100 ms) | |
Josephson sign (notching near the nadir of the S wave) |
Ventricular tachycardia can be a life-threatening rhythm and must be quickly identified and treated. Description of ventricular tachycardia should be based on morphology: monomorphic versus polymorphic. The duration also should be noted: nonsustained versus sustained.
Key electrocardiographic features of ventricular tachycardia include a very wide QRS complex (more than 160 ms), concordance, AV dissociation, fusion beats, and capture beats. Other features, such as Brugada syndrome and Josephson sign, have been helpful in identifying ventricular tachycardia. Ventricular tachycardia can further degrade into ventricular fibrillation, which is represented by chaotic, disorganized electrical activity (Fig. 3.4).
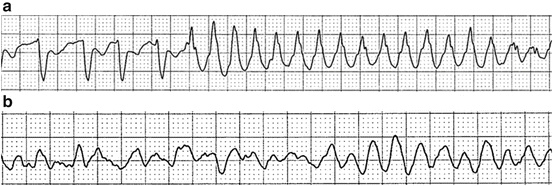

Fig. 3.4
A 68-year-old man with aplastic anemia and severe hypokalemia (K: 2.3) and hypomagnesemia (Mg: 1.5) experienced spontaneous ventricular tachycardia (a) that rapidly degraded into ventricular fibrillation (b)
Patients with cancer require special consideration owing to the risk of QT-interval prolongation and torsades de pointes resulting from the use of both chemotherapeutic agents and adjunct medications.
Treatment of Ventricular Tachycardia/Fibrillation
Treatment of hemodynamically significant ventricular tachycardia and ventricular fibrillation should follow Advanced Cardiovascular Life Support standard guidelines established by the American Heart Association (AHA). If the patient is hemodynamically compromised, emergent electrical defibrillation should be performed. Antiarrhythmic therapy should be administered under the supervision of a cardiologist when possible. First-line antiarrhythmic therapy includes IV β-blockers, amiodarone (Class III antiarrhythmic), and procainamide (Class Ia antiarrhythmic). IV lidocaine (Class Ib antiarrhythmic) may be reasonable, particularly in the setting of myocardial ischemia or infarction. For torsades de pointes, first-line treatment includes long-acting β-blockers and IV magnesium sulfate (typical dose, 2-g IV push). Secondary medications include isoproterenol in patients with torsades de pointes without prolonged QT intervals. Mexiletine (Class Ib antiarrhythmic) and flecainide (Class Ic antiarrhythmic) may shorten the QT interval and be somewhat effective in patients with prolonged QT intervals (European Heart Rhythm Association et al. 2006).
Ablation therapy may be considered for patients with ventricular tachycardia or fibrillation that is refractory to medical therapy or who are intolerant of it. Use of an implantable cardioverter defibrillator may be indicated in appropriate patients who have survived cardiac arrest or have recurrent syncope despite undergoing medical therapy. Early referral to a cardiac electrophysiologist is recommended for further evaluation and treatment of recurrent or symptomatic ventricular arrhythmias .
Treatment of SVT with Aberrancy
Therapy for SVT with aberrancy should follow the recommendations for narrow QRS complex tachycardia described above.
ACS
ACS is a major cause of morbidity and mortality in the developed world and accounts for approximately 2.5 million hospitalizations worldwide and more than 1.4 million hospitalizations in the United States annually. Investigators have performed significant research in this field. However, little remains known about ACS in cancer patients. Working in a large cancer center enables our involvement in caring for a large number of cancer patients with ACS, but the uniqueness of the patient population and diversity of cancer treatment limit the capacity to generalize and provide solid guidelines regarding this subgroup of patients with ACS.
Definition
ACS includes a continuum of clinical presentations covered by the following range of diagnoses: unstable angina (UA) , non-ST-elevation myocardial infarction (NSTEMI) , and ST-elevation myocardial infarction (STEMI) . UA and NSTEMI are also called non-ST-elevation ACS to distinguish them from STEMI.
The symptoms of UA result from myocardial ischemia caused by an underlying imbalance between supply and demand of myocardial oxygen. UA is defined as angina pectoris that can present with one of three features: (1) occurs at rest or with minimal exertion and usually lasts more than 20 min (if not interrupted by treatment with nitroglycerin); (2) new-onset, severe, frank pain; and (3) a crescendo pattern (more severe, prolonged, or increased pain). Some patients with prolonged pain at rest have evidence of myocardial necrosis according to their levels of cardiac serum markers (creatine kinase muscle-brain fraction, troponin T or I, or both) and have an NSTEMI.
Pathogenesis
The most common cause of UA and NSTEMI is plaque rupture and coronary thrombosis with compromise of blood flow to a region of viable myocardium. Fissure or rupture of these plaques and consequent exposure of core constituents (lipid, smooth muscle, and foam cells) to the bloodstream leads to the local generation of thrombin and deposition of fibrin. This in turn promotes platelet aggregation and adhesion and intracoronary thrombus formation. UA and NSTEMI are generally associated with white, platelet-rich, and only partially occlusive thrombi. In many cases, this myonecrosis is thought to result from downstream microembolization of platelet aggregates from a ruptured unstable plaque. In contrast, patients with an STEMI (or Q-wave myocardial infarction) have red, fibrin-rich, and more stable occlusive thrombi. Acute coronary occlusions leading to STEMI tend to cluster in predictable “hot spots” within the proximal third of the coronary arteries. Other less common causes of UA and NSTEMI are dynamic obstructions (e.g., coronary spasm in patients with Prinzmetal angina), progressive mechanical obstructions, inflammation, infections, and secondary UA as a result of mismatch between supply and demand (e.g., anemia).
Diagnosis
The initial diagnosis of ACS is based on history, risk factors, and echocardiography findings. The patient’s history is of the utmost importance in the recognition of acute myocardial infarction. The typical presentation may not be typical in critically ill cancer patients, and physicians should have high indices of suspicion with any patient who presents with new congestive heart failure (CHF) , ventricular arrhythmia, hypotension, heart murmur of mitral insufficiency, or systemic embolic events or who were resuscitated from apparent sudden death. Serial serum measurements of cardiac enzymes and serial ECGs should be performed for such patients.
As many as half of all cases of ACS are clinically silent and, consequently, go unrecognized by the patient. In addition, elderly patients may only present with altered mental status.
Risk factors for ACS include male sex, diabetes mellitus, smoking history, hypertension, advanced age, hypercholesterolemia, and prior cerebrovascular accident or peripheral vascular disease in general .
UA and NSTEMI are closely related conditions with similar clinical presentations. Distinction between them depends on whether the ischemia is severe enough to cause myocardial necrosis that can lead to the release of detectable quantities of intramyocardial biomarkers. Cardiac troponin I and T are the preferred biomarkers, as they are more specific and reliable than creatine kinase or its isoenzyme creatine kinase muscle-brain fraction.
ECGs are similar in patients with UA and NSTEMI and can have transient or persistent ST-segment depressions and T-wave flattening or inversion in the ECG leads reflecting the location of the myocardium in jeopardy. Also, patients with metastatic cancer involving the heart may have ECG abnormalities that resemble those seen in patients with myocardial ischemia.
Physical examination may exclude important differential diagnoses, such as chest wall lesions, irradiation burns, masses, pleuritis, pericarditis, and pneumothorax. It also may reveal evidence of ventricular failure and hemodynamic instability .
Early Risk Stratification
The Thrombosis in Myocardial Infarction (TIMI) risk score is a commonly used risk-stratification tool. The predictable variables in this score are (1) age greater than 65 years, (2) more than three conventional risk factors for coronary artery disease, (3) known coronary artery stenosis greater than 50 %, (4) ST-segment deviations on presenting ECGs, (5) more than two anginal events within the prior 24 h, (6) use of aspirin within 7 days, and (7) elevated serum cardiac marker levels.
Management
Tailoring treatment of ACS to risk in cancer patients not only ensures that patients who will benefit the most receive aggressive treatment but also prevents potentially hazardous treatment in those with poor prognoses. The treatment approach should take into account the status of the patient’s cancer to avoid unnecessary procedures or actions that can delay cancer treatment. At the other end of the spectrum, suboptimal treatment of ACS may significantly decrease the patient’s ability to complete cancer treatment and survive the disease. Initial medical treatment of ACS includes bed rest and use of oxygen and opiate analgesics to relieve pain, anti-ischemic medications, and antiplatelet/antithrombotic drugs.
Anti-Ischemic Therapy
Class I recommendations for anti-ischemic therapy include bed rest, use of supplemental oxygen as needed, and sublingual/IV administration of nitroglycerin for ongoing symptoms in the absence of contraindications.
β-blockers are central to treatment of ACS, which is reflected in the 2012 and 2013 American College of Cardiology (ACC) Foundation/AHA guidelines (2012 Writing Committee Members et al. 2012; O’Gara et al. 2013). Oral β-blockade for UA and NSTEMI is a Class Ia recommendation in the absence of heart failure, a low output state, increased risk of cardiogenic shock, age greater than 70 years, systolic blood pressure less than 120 mm Hg, sinus tachycardia greater than 110 bpm, heart rate less than 60 bpm, or any other relative contraindication. IV β-blockade is now reserved for specific indications, such as ongoing rest pain, especially with tachycardia or hypertension. Patients at lower risk are those who tend to gain the most from β-blockade. IV β-blockade is specifically avoided in patients with heart failure, hypotension, or hemodynamic instability.
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers are recommended as Class I therapy for UA and NSTEMI in patients with pulmonary congestion or a left ventricular ejection fraction less than 0.40 in the absence of hypotension. ACE inhibitors improve short- and long-term survival after myocardial infarction complicated by left ventricular dysfunction (Studies of Left Ventricular Dysfunction, Survival and Ventricular Enlargement, and Trandolapril Cardiac Evaluation trials) and should be administered (preferably orally) within 24 h and continued upon discharge unless contraindicated. In patients with intolerance to ACE inhibitors, angiotensin-receptor blockers can be considered as alternative therapy.
Other Class I recommendations include (1) use of nondihydropyridine calcium-channel blockers (verapamil or Cardizem) in cases of β-blocker intolerance with an absence of contraindications and (2) discontinuation of nonsteroidal anti-inflammatory drugs (both nonselective and cyclooxygenase-2–specific agents) owing to increased risk of ischemic events and myocardial rupture.
Antiplatelet/Antithrombotic Therapy
Platelet aggregation and thrombus formation play key roles in the development of ACS. Recent advances in treatment, such as low-molecular-weight heparin, glycoprotein (GP) IIb/IIIa inhibitors, and clopidogrel, and the increasingly safe and widespread use of percutaneous coronary intervention (PCI) have raised questions about optimal antiplatelet/antithrombotic management.
Antiplatelet Therapy
Antiplatelet therapy for ACS is achieved by balancing the extent of platelet activation using a combination of antiplatelet drugs having complementary actions: aspirin, thienopyridines, and GP IIb/IIIa inhibitors.
Aspirin
Use of aspirin is the cornerstone of antiplatelet therapy and irreversibly acetylates platelet cyclooxygenase-1, thereby blocking the production of prostaglandin G2 and thromboxane A2. In patients with ACS, aspirin should be administered as soon as possible and continued indefinitely unless the patient is intolerant of it (Class I). Patients with platelet counts as low as 17 k/mL have taken aspirin as antiplatelet therapy, but no therapeutic recommendations can be made until more data on aspirin are available (Yusuf et al. 2010).
Adenosine Diphosphate Receptor Antagonists
Ticlopidine and clopidogrel inhibit platelet activation by irreversibly blocking surface adenosine diphosphate receptors. Thienopyridines are recommended as Class I therapy for UA and NSTEMI in an initial noninvasive strategy or as an alternative to GP inhibitors in an early invasive pathway. Ticlopidine is infrequently used owing to the rare but potentially life-threatening side effects of severe neutropenia and thrombotic thrombocytopenic purpura. Clopidogrel administration at a loading dose of 300–600 mg followed by a maintenance dose of 75 mg daily is a Class I recommendation as both a conservative approach and as an alternative to GP IIb/IIIa receptor inhibitors in an early invasive strategy. In addition, clopidogrel should be administered to UA and NSTEMI patients who are unable to take aspirin because of hypersensitivity or major gastrointestinal intolerance.
Considering that data suggest that administration of 600 mg of clopidogrel prior to and after PCI is beneficial, the debate now extends to the optimal timing of clopidogrel use in patients with UA or NSTEMI. Early treatment with clopidogrel reduces the incidence of early ischemic events, benefitting those who ultimately receive PCI, but increases the risk of bleeding if the patient’s coronary anatomy is unknown. Coronary artery bypass grafting (CABG ) is a possibility in these patients. About 50–60 % of patients presenting with ACS will receive PCI, and 8–20 % are considered for CABG. Early identification of patients who may need urgent CABG using TIMI risk score tools may help in identifying those who should not receive early 600-mg loading doses of clopidogrel, minimizing the bleeding risk in those proceeding to CABG, and preserving benefit for the majority of patients needing PCI. Although the optimal timing, dosing, and duration of treatment with clopidogrel remain undetermined, our approach in cancer patients includes an early TIMI score, and if bypass is not considered, we load patients with 600 mg of clopidogrel.
Proton pump inhibitors are often administered to patients in combination with thienopyridines to help reduce the risk of bleeding after ACS or PCI. Their use is even greater in cancer patients. Several studies have demonstrated that proton pump inhibitors, especially omeprazole, can diminish the antiplatelet effects of clopidogrel. However, at present, scarce data demonstrate a definitive interaction between proton pump inhibitor use and the clinical benefit of clopidogrel (Gilard et al. 2008).
Ticagrelor is an oral, reversible, direct-acting inhibitor of the adenosine diphosphate receptor P2Y12 that has a more rapid onset and more pronounced platelet inhibition than clopidogrel. In patients having ACS with or without ST-segment elevation, treatment with ticagrelor produced a lower rate of death from vascular causes, myocardial infarction, or stroke than did treatment with clopidogrel without an increase in the rate of overall major bleeding but with an increase in the rate of non-procedure-related bleeding.
The optimal ticagrelor dosing strategy as determined according to the agent’s pharmacokinetic and pharmacodynamic profile is a loading dose of 180 mg followed by 90 mg orally twice a day. Within 30 min, a ticagrelor loading dose of 180 mg has resulted in roughly the same level of platelet aggregation inhibition as that achieved 8 h after administration of a clopidogrel loading dose of 600 mg.
Ticagrelor blocks reuptake of adenosine by red blood cells, which leads to cardiovascular benefit via reduced blood pressure, improved coronary flow, or protection against reperfusion injury. This explains why some patients experience bradycardia and dyspnea with the use of ticagrelor. Use of ticagrelor is a promising approach to the prevention of cardiovascular events in patients with ACS.
Anticoagulation
The heparins include unfractionated heparin (UFH) , low-molecular-weight heparin, and fondaparinux, a synthetic heparin pentasaccharide that primarily acts by neutralizing factor Xa.
UFH, the prototype of all heparin derivatives, is a standard antithrombotic therapeutic for ACS in all patients regardless of the treatment approach. Parenteral anticoagulation with IV UFH or subcutaneous low-molecular-weight heparin should be added to antiplatelet therapy with aspirin or a thienopyridine (Class I recommendation). UFH is usually administered by IV injection followed by infusion, starting with weight-adjusted doses. The activated partial thromboplastin time is used to monitor anticoagulation in most circumstances, although the activated clotting time is used when higher intensity anticoagulation is required (e.g., during PCI, with cardiopulmonary bypass).
Plaque disruption with resultant platelet activation and leukocyte-platelet aggregation is the pathophysiologic process common to both ACS and PCIs. Treatment with low-molecular-weight heparins has caused less platelet activation than that with unfractionated heparin.
Enoxaparin has demonstrated advantages over UFH in low- to moderate-risk patients with non-ST-elevation ACS treated using a conservative strategy. Enoxaparin is a safe and effective alternative to UFH with the advantages of convenience and a trend toward producing a lower rate of nonfatal myocardial infarction with a modestly excessive risk of bleeding. Enoxaparin is preferable to UFH as an anticoagulant in patients with UA or NSTEMI in the absence of renal failure and/or need for CABG (Class IIa recommendation). PCI can be performed safely in patients with UA or NSTEMI who have received the typical dose of enoxaparin.
Physicians have used dalteparin in an early invasive strategy in moderate- to high-risk patients with non-ST-elevation ACS, resulting in sustained benefit at 5 years of follow-up. Dalteparin appears to be safe in combination with abciximab in patients with UA undergoing coronary intervention. Abciximab-based therapy during coronary interventions rapidly reduces the amount of platelet degranulation and number of leukocyte-platelet aggregates.
Fondaparinux is a synthetic heparin pentasaccharide that acts via antithrombin to exclusively neutralize factor Xa. Regimens using enoxaparin, UFH, or fondaparinux have established efficacy in patients in whom a conservative strategy is selected. In patients in whom a conservative strategy is selected and who have an increased risk of bleeding, fondaparinux is preferable. Administration of fondaparinux is not recommended prior to or during primary PCI in patients with STEMI owing to an increased risk of guiding-catheter thrombosis. Patients with UA, NSTEMI, or STEMI undergoing any PCI should not receive fondaparinux as the sole anticoagulant. Use of an anticoagulant with antithrombin activity (e.g., UFH) is recommended as adjunct therapy with PCI even if the patient received prior treatment with fondaparinux.
GP IIb/IIIa Inhibitors
The typical pharmacotherapeutic strategy for patients with non-ST-elevation ACS has been an intensive combination of aspirin, clopidogrel, and GP IIb/IIIa inhibitors (abciximab, tirofiban, and eptifibatide) along with an antithrombin (UFH or low-molecular-weight heparin). Recent clinical trials challenged the role of GP IIb/IIIa-based strategies and suggested new treatment options that differ by omitting GP IIb/IIIa-based antiplatelet therapeutics. A consequence of the resulting data has been a variety of pharmacotherapeutic regimens that differ from the ACC/AHA guidelines. For example, the threshold for administering GP IIb/IIIa inhibitors is even higher in patients receiving ongoing treatment of cancer (chemotherapy, radiation therapy, or surgery), which is driven mainly by increased risk of bleeding.
Because the pharmacotherapeutic approaches for non-ST-elevation ACS requiring PCI are complicated by a myriad of evolving antiplatelet strategies that have existed experimentally and outside our current evidence-driven guidelines, the optimal use of GP IIb/IIIa receptor antagonists involves identifying the appropriate patients, window for therapy, drug, and dosing. Heterogeneity in clinical trials has borne a mixture of data suggesting both benefit and equivalence, making interpretation difficult for both clinical and interventional cardiologists. High-risk patients with non-ST-elevation ACS requiring PCI are most likely to benefit from treatment with GP IIb/IIIa receptor antagonists when they have ongoing ischemia, dynamic ECG changes, and troponin positivity owing to unstable plaque with active inflammation .
When platelets are activated, the surface GP IIb and IIIa undergo a change in conformation that increases their affinity for binding to fibrinogen and other ligands, resulting in platelet aggregation. The platelet GP IIb/IIIa receptor antagonists act by occupying the receptors and preventing fibrinogen from binding, thereby preventing platelet aggregation. Experimental and clinical studies have suggested that occupancy of at least 80 % of the receptor population and inhibition of platelet aggregation to adenosine diphosphate by at least 80 % result in potent antithrombotic effects. The various GP IIb/IIIa antagonists have significantly different pharmacokinetic and pharmacodynamic properties. Available data suggest that the combination of eptifibatide and clopidogrel provides greater antiplatelet activity than does clopidogrel alone. How this translates to improved clinical outcomes remains to be evaluated.
The Intracoronary Stenting and Antithrombotic Regimen trials examined the necessity of treating coronary artery disease with GP IIb/IIIa inhibitors in various patient populations and settings and with various pharmacotherapeutic regimens. GP IIb/IIIa receptor blockade limits the ischemic complications of PCI across all indications, among various devices, and with multiple anticoagulation approaches using a variety of agents. Future guidelines should provide more specific direction regarding risk stratification in an era in which GP IIb/IIIa receptor blockade and clopidogrel may be used in concert in patients with non-ST-elevation ACS who undergo PCI .
Heparin-Induced Thrombocytopenia Patients
In 10 % of patients receiving treatment with UFH for 5 days or more, heparin-induced thrombocytopenia is known to develop and is usually reversible after heparin withdrawal. Alternative agents used effectively in patients with heparin-induced thrombocytopenia include lepirudin, argatroban, bivalirudin, and danaparoid, although the last agent is not available in North America. Fondaparinux is used in a small number of patients with heparin-induced thrombocytopenia and generally appears to be safe. (Please refer to the Hematologic Emergencies chapter for more discussion on heparin-induced thrombocytopenia .)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree