Malignancies causing peritoneal surface disease (PSD)
Primary Peritoneal
Mesothelioma
Locoregional metastasis
Appendiceal
Colon
Ovarian
Gastric
Small bowel
Sarcoma
Distant metastasis
Breast
Other
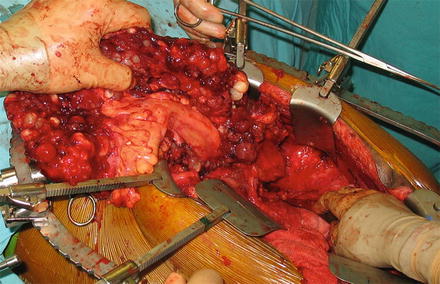
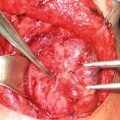
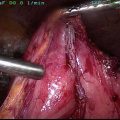
Fig. 1
Intraoperative image of peritoneal surface disease . Patient’s head is at left. Note the large tumor nodules throughout the omentum, which is held in the surgeon’s hand
In most cases, peritoneal carcinomatosis is diagnosed incidentally during surgical exploration, evaluation of abdominal pain, or on radiographic imaging for other indications. Unfortunately, these patients have a dismal prognosis without treatment, with median survival reported between 3 and 7 months [1]. This chapter describes the staging, treatment, and current patient outcomes in peritoneal carcinomatosis as well as controversies in the management of this disease.
Staging of Peritoneal Surface Malignancies
Imaging
The most commonly used imaging modality for both diagnosis and preoperative evaluation is contrast enhanced computed tomography (CT) scan of the chest, abdomen, and pelvis. The first objective evaluation of this modality in 1993 by Sugarbaker and colleagues demonstrated an overall sensitivity of 79 % in detecting peritoneal lesions [2]. In that study, sensitivity was lowest in the pelvis and decreased with decreasing tumor volume, with a sensitivity of only 28 % obtained for tumor nodules less than 5 mm in size. Although CT technology has progressed since 1993, the sensitivity for gross detection of peritoneal lesions remains similar, with more recently described overall sensitivity of detection rates of 60–76 % [3]. Again, the sensitivity of CT scans is highly dependent on implant size, with sensitivities of upwards of 94 % for nodules >5 cm in size [4]. Despite its shortcomings, CT is appropriate for the detection of solid organ involvement, retroperitoneal spread, overall operability, and prognosis, making it the continued imaging modality of choice [5, 6] (Fig. 2).
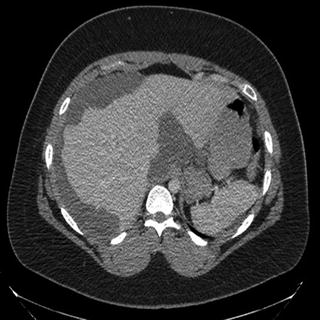

Fig. 2
Contrast enhanced computed tomography of a patient with large volume pseudomyxoma peritonei. Tumor encases the lateral liver and porta hepatis. A smaller amount of disease is deposited on the spleen. With kind permission from Springer Science + Business Media: Surgical Oncology, Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy, 2015, Randle RW et al., Figure 27.2
Magnetic resonance imaging (MRI) with both oral and intravenous contrast has been reported to have an overall sensitivity of detection of peritoneal implants of between 84 and 100 % [7, 8]. In many patients with peritoneal surface malignancies, prior operative management makes MRI a poor imaging modality as it cannot discern between postoperative scar and peritoneal implants, resulting in a high false positive rate in this group of patients [9]. Overall, the increased cost, time, and lack of prognostic significance of MRI have not made it a preferred imaging choice for PSD.
The role of positron emission tomography (PET) in patients with peritoneal surface dissemination is mainly to detect extra-abdominal disease in planning treatment. Sensitivity is reported at approximately 10 % in low volume disease. Unlike contrast CT, which has a specificity of >90 %, PET specificity remains approximately 42 % in patients with low volume disease [10, 11]. In addition, not all histological subtypes of carcinomas that present as diffuse PSD have sufficient glucose uptake of 18F-FDG. Despite these shortcomings, PET may be warranted to rule out extra-abdominal disease in patients being considered for aggressive surgical therapy. PET imaging is of limited utility for patients with low grade appendiceal disease [12].
Staging
Given the aforementioned findings, the two primary staging algorithms are based on CT scan and operative findings. The Peritoneal Carcinomatosis Index (PCI) is the most widely used staging system for peritoneal carcinomatosis. This system divides the abdomen into nine regions and the small bowel into four regions. A score is assigned to each region based on the amount of tumor present. A score of 0 (no tumor), 1 (tumor up to 0.5 cm), 2 (tumor up to 5 cm), or 3 (tumor >5 cm) is applied to each region and the scores for the 12 regions are then tabulated to give a final score [13] (Fig. 3). The PCI has become the most widely cited scoring system as it can be used both pre and postoperatively, and correlates with likelihood of complete resection and prognosis [14–16]. The Gilly Carcinomatosis Staging Scale is an alternative staging system with scoring ranging between Stage 0 to Stage 4 [17]. Stage 0 is applied for patients with no macroscopic disease, Stage 1 designates localized tumor implants less than 0.5 cm in diameter, Stage 2 disease represents non-localized tumor implants less than 0.5 cm, Stage 3 identifies implants 0.5–2 cm, and Stage 4 represents any implants greater than 2 cm in diameter. As with the the PCI, higher stage correlates with worse prognosis [18–20].
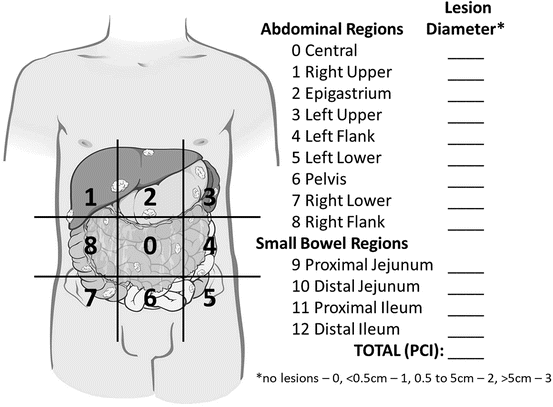

Fig. 3
Schematic for calculating the peritoneal carcinomatosis index (PCI) staging system. Scores based on lesion size for each of nine abdominal regions plus four small bowel regions are added together to reach the PCI. With kind permission from Springer Science + Business Media: Surgical Oncology, Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy, 2015, Randle RW et al., Figure 27.3
Surgical Staging
Laparoscopic staging has been suggested as a more sensitive form of staging than the imaging modalities above over the last 10 years. Denzer et al. conducted a comparison of CT scan versus “minilaparoscopy” in the detection of peritoneal surface malignancies. They reported that diagnostic laparoscopy detected PSD in 100 % of the treated cases, whereas only 47.8 % had been revealed by a CT scan previously performed on the same patients [21]. Taking this further, Valle and colleagues described the use of 2–3 port video laparoscopy in assessing PCI and resectability in 97 patients [22]. They reported that 16/97 (16 %) patients presented with disease that was deemed unresectable based upon laparoscopic staging, while two patients were under-staged by laparoscopy. They also noted that while there was no tumor seeding of the trocar sites, there was a 2 % superficial soft tissue infection of the trocar sites. This study, however, did not include evaluation of preoperative CT scans to assess whether all of the enrolled patients would have been eligible for laparotomy by radiographic criteria. This question was more directly addressed by Pomel and colleagues in an assessment of 11 patients of uncertain resectability [23]. Laparotomy was avoided in three patients (27 %); one patient was deemed to have completely resectable disease at laparoscopy could not be completely debulked (9 %); and seven patients underwent successful resections (63.6 %). On the other hand, 20 % of patients who went directly to laparotomy in their comparative study could not undergo a complete resection. Based on these limited studies, it remains to be determined whether laparoscopy has a role in determining staging and potential resectability in patients for whom imaging is inconclusive. At present, the use of laparoscopy remains an area of controversy.
Current Treatment Modalities
Currently, the best management of patients with peritoneal surface disease remains an area of debate. Options for treatment include systemic chemotherapy, surgical debulking alone, or surgical debulking with adjuvant intraperitoneal chemotherapy. These approaches are described below.
Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy (CRS/HIPEC)
Surgical resection is recommended for removal of the primary lesion, and peritoneal debulking is undertaken for disseminated peritoneal surface disease. The cytoreduction procedure removes macroscopic tumor deposits but if microscopic disease is not addressed, traditionally 90 % of patients developed disease progression and death, typically from bowel obstruction. In 1980 a method of combining debulking surgery with heated intraperitoneal chemotherapy was first described [24]. The administration of cytotoxic chemotherapy directly into the peritoneal cavity has been shown to achieve higher local concentrations at the site of disease than could be achieved systemically [25]. The addition of heat was found to be synergistic with the chemotherapy [9]. As a complete technique, CRS/HIPEC targets both macroscopic and microscopic disease.
The surgical technique involves both the cytoreduction and the perfusion step. Cytoreduction is the key intervention, and is undertaken in a manner similar to exploratory laparotomy. If still in place, the primary lesion is removed as well as any involved organs. Tumor deposits are then stripped from peritoneal surfaces, including the abdominal wall and diaphragm. Bowel and organ resection may be undertaken if the peritoneum is unable to be stripped. Anastomoses can be performed prior to or following HIPEC, while stomas are created following the chemoperfusion. Following cytoreduction, perfusion is performed by either a closed abdomen or open or “coliseum” technique. Inflow and outflow catheters are placed through the abdominal wall and connected to the heat exchanger and pump of the perfusion circuit. In the closed technique, the abdomen is temporarily closed with a running watertight suture. In the open or coliseum approaches, a temporary plastic sheet is sewn onto the abdomen. Perfusion is generally maintained for 30–120 min dependent on the individual center’s protocol for the tumor type. Following perfusion, the cannulas and/or plastic sheet are removed, stomas created if required, and the abdomen is closed.
The goal of CRS/HIPEC is complete surgical removal of macroscopic disease and chemical destruction of microscopic disease by the chemotherapeutic agent. Disease-free progression and survival have been correlated with the completeness of resection, as judged by the surgeon. Two classification systems are utilized in the reporting of the completeness of resection in both clinical documentation and research. The R status of resection (from the AJCC staging manual) or the CC score for completeness of cytoreduction can be used as shown in Table 2. Complete cytoreduction of all macroscopic disease is designated as R0 or R1 on the R scale and CC-0 on the CC scale. R0 versus R1 allows the distinction of negative versus positive margins on pathology. R2 resection or >CC-0 indicate residual macroscopic disease. Regardless of the origin of the tumor, the resection status is the main independent predictor of survival across multiple studies [26–31].
Table 2
Comparison of cytoreductive surgery scoring systems
Residual disease (cm) | Residual disease (R) status | Completeness of cytoreduction (CC) score |
|---|---|---|
0 | R0-Negative margins on final pathology | CC-0: No visible disease following cytoreduction |
R1-Positive margins on final pathology | ||
0.25 | R2a | CC-1 |
0.5 | CC-2 | |
>0.5–2 | R2b | |
>2–2.5 | R2c | |
>2.5 | CC-3 |
In addition to resection status, patient factors prior to surgery have a significant impact on overall survival. Preoperative performance status is a significant prognostic factor. Patients with ECOG scores of 0 or 1 have been shown to have better outcomes after CRS/HIPEC then those with scores of 2 or 3. For example, in one study of patients with PSD from a variety of primaries, median survival was 21.7 months with an ECOG of 0–1 and 9.5 months with ECOG 2–3 [32].
Many chemotherapeutic agents have been utilized for intraperitoneal chemotherapy. At present, the most widely utilized agents are oxaliplatin and mitomycin C. A prospective randomized controlled trial of these two agents is ongoing for appendiceal cancer at Wake Forest University and should complete accrual in 2015. Randomized trials for intraperitoneal chemotherapeutic agents are otherwise lacking in this setting, and agents have historically been chosen based on large molecular weight conveying an ability to achieve higher intraperitoneal concentrations with low systemic absorption and associated toxicities.
Systemic Chemotherapy
Peritoneal surface disease represents a unique challenge for systemic chemotherapy. It has long been understood that the permeability of the peritoneal cavity to many systemic chemotherapy drugs is less than the plasma clearance, therefore allowing little of the systemically delivered drug to reach the PSD tumor nodules [33–35]. The capacity of the peritoneal cavity to serve as a barrier to drug transport is thought to lie mainly in the transport properties of the blood capillary wall and interstitial matrix [36]. This is supported by the fact that surgical peritonectomy of the peritoneal membrane itself does not alter intraperitoneal concentrations of chemotherapeutic agents [37]. Given this information, it is not surprising that the clinical response of patients with PSD to systemic chemotherapy is modest at best. As will be discussed below, the most widely studied patients with PSD undergoing systemic chemotherapy are those with PSD from colorectal carcinoma (CRC). In this select group, median survival is approximately 19–22 months with modern systemic chemotherapy combined with biologics, with 5 year survival rates <5 % [38, 39]. The role of systemic chemotherapy, if applicable, will be discussed within each disease specific section below.
Overview of Peritoneal Carcinomatosis of Select Cancers
Appendiceal Neoplasms
Epithelial neoplasms of the appendix represent a rare disease and are estimated to be diagnosed in approximately 1 % of all appendectomy specimens [40]. Those not diagnosed in appendectomy specimens often present late with peritoneal surface disease due to the nonspecific nature of appendiceal neoplasm symptoms. In peritoneal surface disease, the lumen becomes obstructed by tumor, which is often mucin producing, and subsequent rupture leads to peritoneal dissemination.
Pseudomyxoma peritonei , or malignant mucinous ascites, develops in approximately 10 % of patients with an epithelial appendiceal neoplasm. In those who develop PSD, patient prognosis correlates with pathologic classification. At present, two potential pathologic classification schemes exist, indicating an area of controversy. The system proposed by Ronnett et al. in 1995 is a staging system consisting of three categories; Diffuse peritoneal adenomucinosis (DPAM) consisting of low-grade tumors with low numbers of mitotic figures and cytologic atypia; peritoneal mucinous carcinomatosis (PMCA) consisting of high-grade tumors and characterized by atypia and more prominent mitotic figures; and, PMCA-I/D marked by intermediate or discordant features [20]. A subsequent study showed that indeed, these categories correlated with both 5 and 10-year survival. Survival for patients with DPAM (75 %, 68 %) was significantly better than that for PMCA-I/D (50 %, 21 %) or PMCA (14 %, 3 %) [41]. More recently, the group at Wake Forest has proposed a two-tiered classification system based upon a pathologic review of 101 patients with mucinous ascites related to primary appendiceal tumors. The 5-year survival for DPAM and PMCA-I/D was found to be similar, at 68 % and 61 %, respectively. PMCA, however, was associated with a statistically significant 5-year survival of 37 % (p = 0.004) [42]. Based upon this information, the two categories proposed were low grade mucinous carcinoma peritonei (MCP-L) and high-grade mucinous carcinoma peritonei (MCP-H). Low grade mucinous carcinoma encompasses DPAM, PMCA I/D, and well differentiated variants of mucinous adenocarcinoma. High grade mucinous carcinoma includes PMCA, histologically moderate or poorly differentiated adenocarcinoma, and cases with signet ring cell components. Categorizing PSD from appendiceal tumors into two categories has remained more consistent with response to CRS-HIPEC as well [43]. Approximately 16 % of low grade lesions are thought to dedifferentiate into higher-grade lesions during the course of the disease [44].
Despite their rarity, outcomes from appendiceal neoplasms have been extensively studied due to their role as the classic indication for CRS/HIPEC. A 2008 consensus statement from the Fifth International Workshop on Peritoneal Surface Malignancy supported CRS/HIPEC as the standard of care for perforated appendiceal tumor with PSD, based on a review of the evidence [45]. Due to the rarity of this entity, randomized studies have not been undertaken directly comparing cytoreduction versus CRS/HIPEC. Cytoreduction alone is known to confer a significant survival benefit. Gough et al. reported 5 years and 10 years survival rates of 53 % and 32 % respectively with serial debulking alone [46]. While no direct comparisons exist, studies investigating survival post CRS/HIPEC have shown 5 year survival rates ranging from 60 to 97 %, with a recent study reporting a 15-year survival rate of 59 % [42, 47–50].
The importance of cytoreduction is emphasized by the fact that the extent of the resection is a large factor in the wide range of survival rates. In a series of 481 appendiceal carcinoma patients, the extent of cytoreduction was independently associated with survival (p < 0.001) with median survival times of 175 months, 73 months, 29 months, and 17 months for R0/1, R2a, R2b, or R2c resections, respectively [51]. This significant correlation of R0/1 resection with increased survival correlates with findings from other studies [50, 52]. The wide range of survival percentages also indicates that survival is likely not dependent on surgery alone, but with multiple patient factors. The largest retrospective study to date on CRS/HIPEC for appendiceal cancer was a recent large multi-institutional study involving 2298 patients from 16 institutions. Multivariate analysis from this study indicated that preoperative PCI score, prior chemotherapy, PMCA histopathologic subtype, incomplete debulking, major (Grade 3, 4) post operative complications, and not including HIPEC were predictors of shorter progression-free survival [29].
The role of perioperative systemic chemotherapy in appendiceal carcinoma remains an area of controversy. An initial study by Sugarbaker and colleagues evaluated 34 patients with PMCA and found a 29 % histopathologic response after 3–6 months of FOLFOX with or without bevacizumab [52]. However, they found that there was no overall survival advantage to neoadjuvant chemotherapy (p = 0.56) in this same population of patients in a follow-up study [53]. Not surprisingly, however, patients who had a histopathological response to neoadjuvant therapy had a significant survival advantage (median survival NR) versus those who did not (median survival 29.5 months, p = 0.034). In addition, perioperative chemotherapy was associated with a lower preoperative PCI (p = 0.003) and decreased number of visceral resections (p < 0.001), but not with completeness of resection (p = 0.78) or complications (p = 0.16). This study excluded patients with low grade disease (MCP-L), classified as DPAM or PMCA I/D. In contrast Chua et al. performed a registry review which implicated prior chemotherapy in the setting of CRS/HIPEC was associated with poorer progression free and overall survival [31]. Our group studied both MCP-L and MCP-H patients and found no improvement in median progression free survival (PFS) in patients with MCP-L treated with perioperative chemotherapy versus those not (29.5 months vs 37 months, p = 0.18). In those patients with MCP-H, postoperative chemotherapy was associated with longer PFS (13.6 months, p < 0.01) than preoperative chemotherapy (6.8 months) or CRS/HIPEC alone (7 months, p = 0.03) [54].
Modern chemotherapy may offer modest benefit in patients with unresectable disease. Similar to the histopathologic response reported in surgical patients, Farquharson et al. reported that 38 % of patients with unresectable PSD from appendiceal carcinoma experienced a reduction in ascites or stabilization of disease with systemic chemotherapy consisting of mitomycin c and capecitabine [55]. Shapiro et al. additionally reported a 55.6 % total disease control rate in patients deemed unresectable and receiving at least two cycles of systemic chemotherapy. Disease control included complete response, partial response, and stabilization of disease in this study [56]. While CRS/HIPEC remains the standard of care in appropriate surgical patients, in patients deemed suboptimal candidates for surgical resection, systemic chemotherapy offers an option for potential disease and symptom control. Further, it clearly has a role in high grade appendiceal cancer with PSD.
Colorectal Cancer (CRC)
Colorectal cancer is the third most common cancer in the United States. Despite the significant improvements in systemic chemotherapy, CRC continues to have high rates of recurrence. While the liver and lung remain the most common sites of recurrence, peritoneal surface disease (PSD) is the only site of recurrence in up to 25 % of patients [56]. In a retrospective study of 3019 patients with CRC, Jayne et al. reported peritoneal surface disease in 349 (13 %) patients. The majority of the patients (214 out of 349) had synchronous metastasis and 58 % of that subset having PSD as the only site of metastasis [57].
PSD from CRC, like those from other primary tumors, was traditionally considered a terminal disease, all too frequently approached with therapeutic nihilism. PSD from CRC has been shown to be somewhat responsive to chemotherapy. Franko et al., compared outcomes of patients with PSD from CRC enrolled in two prospective randomized trials of chemotherapy with those of non-peritoneal metastatic CRC [58]. Both the median overall survival (12.7 months versus 17.6 months) and progression-free survival (5.8 months vs 7.2 months) were inferior for PSD compared to non-peritoneal metastasis for CRC. However, this survival was a marked improvement from the median 6-month survival for PSD noted in the EVOCAPE-1 trial, and it is noteworthy that approximately 3 % of patients were alive 5 years after diagnosis [1]. Furthermore, it substantiated the role of CRS/HIPEC in improving outcomes for PSD from CRC.
The only randomized trial comparing outcomes of CRS/HIPEC with systemic chemotherapy for PSD from CRC was reported in 2003 [59]. The trial involved 105 patients with PSD from CRC randomly assigned to either systemic chemotherapy (5-fluorouracil and leucovorin) or CRS/HIPEC with the same chemotherapy. After a median follow-up of 21.6 months, the median survival was 12.6 months in the systemic chemotherapy arm versus 22.3 months in the CRS/HIPEC arm (p = 0.032). A more recent update of the study, after a median follow-up of 8 years, reported superior progression-free survival (p = 0.020) as well as disease-specific survival (p = 0.028) in the CRS/HIPEC group [60]. Although this study was criticized for the use of 5-fluorouracil and leucovorin as the systemic chemotherapy regimen instead of the now more standard FOLFOX regimen and for including some appendiceal cancers in the cohort, the significant difference in (the more than doubled) survival related to CRS/HIPEC could not be denied. A subsequent retrospective study by Franko et al. showed that, for best outcomes, CRS/HIPEC should be used in conjunction with systemic chemotherapy and not in lieu of it [61]. Encouraged by the solid evidence, a multi-institutional consensus statement was issued by an international peritoneal surface group, delineating the treatment algorithm for PSD from CRC with particular emphasis on CRS/HIPEC in 2007 [62].
Since that landmark trial, several studies/centers have reported their outcomes with CRS/HIPEC for CRC‐related PSD [63]. Glehen et al. reported a median survival of 19 months after median follow-up of 53 months with 5 year survival of 31 % in a multi-institutional study involving 506 patients from 28 international centers [64]. Several other studies have corroborated these improved outcomes associated with CRS/HIPEC [65, 66]. Based on these encouraging results, a multi‐institutional consensus statement was issued proposing guidelines regarding the indication and technique of CRS/HIPEC for PSD from CRC [67].
Similar to outcomes of CRS/HIPEC for other primary malignancies, several prognostic factors have been identified for PSD from CRC. These include completeness of cytoreduction/residual disease (R) score, performance status of the patient and PCI score. The impact of PCI score on outcome of PSD for CRC is particularly well documented. Sugarbaker showed a 50 % 5‐year survival for PCI < 10 which dropped to 20 % for PCI between 11 and 20. There were no survivors after 5 years amongst patients with PCI >20 [68]. The PCI is also related to the completeness of cytoreduction scores (R or CC); with the PCI and cytoreductions scores being inversely related.
Progressively improving outcomes associated with CRS/HIPEC for PSD for CRC has encouraged more aggressive surgical approaches in quest of even better outcomes. Although synchronous resection of liver metastasis at the time of CRS/HIPEC for PSD from CRC was initially noted to have negative prognostic value, subsequent studies have shown similar outcomes for CRS/HIPEC with and without liver metastatectomy. Chua et al. reported a 2-year survival rate of 68 % in the non-liver metastatic group versus 65 % for the liver metastatic group after complete cytoreduction/metastatectomy [69]. Their results are consistent with studies from Elias et al. and Kianmanesh et al. both of which showed no difference in survival between the two groups [70, 71]. At the 5th International Workshop on Peritoneal Surface Malignancy, it was recommended that in carefully selected patients it was feasible and potentially beneficial to perform CRS/HIPEC with simultaneous metastatectomy provided there were three or fewer liver metastases [69, 72]. This presumed that all hepatic lesions were resected or ablated at the time of surgery and that the hepatic resections/ablations could be performed without lobar hepatectomy.
Ovarian Neoplasms
Epithelial ovarian cancer (EOC) affects approximately 22,000 women annually in the United States, resulting in approximately 15,000 deaths per year [73]. Despite advances in treatment, 5 year survival rates for women with advanced cancer remain less than 50 %. The presenting symptoms of EOC may remain vague and thus diagnosis is often at a late stage. Advanced disease is often confined to the peritoneal cavity, with PSD being a substantial clinical problem in these women [74].
Munnell’s early description of the benefit of “maximum surgical effort” in EOC patients belays the early history of cytoreduction [75]. In his 1968 report, he showed survival benefit for cytoreduction in EOC patients with intraperitoneal disease, which we would now refer to as PSD. Since that time, the definition of “maximum surgical effort” in EOC has evolved. While standards of CRS for EOC at one time called for removal of all disease to <2 cm dimension of the largest lesion, this has now evolved to a standard of <1 cm [76, 77]. A large meta-analysis of 6885 patients found maximal cytoreduction to be an independent predictor of survival among patients with stage III or IV ovarian carcinoma [78].
While CRS is accepted as standard of care, the role of CRS/HIPEC in EOC remains debated. Randomized clinical trials have shown the benefit of the addition of intraperitoneal chemotherapy to CRS. Armstrong et al. randomized 415 patients with Stage III ovarian cancer who had undergone maximal CRS to <1 cm to receive either IV paclitaxel and cisplatin or IV paclitaxel with intraperitoneal cisplatin. The addition of intraperitoneal cisplatin was associated with longer progression free survival (23.8 months vs 18.3 months, p = 0.05) and overall survival (65.6 months vs 49.7 months, p = 0.03) [79]. While this study referred to catheter based early post-operative intraperitoneal chemotherapy (EPIC), studies have also reviewed the role of CRS/HIPEC in EOC. A systemic review of CRS/HIPEC outcomes for PSD from ovarian cancer included 19 studies and was published in 2009 by Chua et al. [80]. They found a wide range of reported outcomes, with 5 year survival ranging from 12 to 66 %. Despite prior descriptions of increased Grade 3/4 complications with intraperitoneal cisplatin, peri-operative morbidity (0–40 %) and mortality (0–10 %) were similar to that reported for CRS/HIPEC in other cancers.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree