Carcinomas of the Head and Neck
Frank E. Mott
Ibrahim Ebada Sadek
Josh David Simmons
I. INTRODUCTION
Data from the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) program estimate more than 1.6 million new cancer cases in 2014. Cancers of the head and neck (HNCs) account for 55,000 or 3.3% of those. The majority of HNCs are in the oral cavity and pharynx (oropharyngeal cancers [OPCs]) accounting for 42,000, with cancers of the larynx the next most common with 12,600.1 The common anatomic sites of HNCs include the oral cavity, nasal cavity, and pharyngeal cavity with subsites defined for nasopharynx, oropharynx, and hypopharynx, and the laryngeal-epiglottic region (considered part of the hypopharynx) (Fig. 6.1). Brain tumors and other cancers of the central nervous system, head and neck lymphomas, and thyroid cancers are considered separately.
The commonly associated causes of squamous cell carcinoma of the head and neck (HNSCC) have been tobacco use in all forms and excessive alcohol consumption. In addition, some viral etiologies have been defined including the Epstein-Barr virus (EBV) and its association with nasopharyngeal carcinomas (NPCs)2; and, in recent years, the human papillomavirus (HPV) and its correlation with OPCs, especially the base of tongue and tonsillar regions.3,4 Eighty to ninety percent are squamous cell histology, with the remainder composed of adenocarcinoma, mucoepidermoid, and adenoid cystic histologies; these latter types are seen mostly in salivary glands.
A. Presenting symptoms
Presenting symptoms in HNC vary depending on the primary site and can be sometimes vague and confused with benign etiologies such as sinusitis or infectious pharyngitis. A heightened level of suspicion, especially in a patient with obvious risk factors such as smoking, is warranted. Vocal hoarseness is a common complaint in patients with laryngeal cancer. Ear pain or persistent
nasal congestion could indicate a nasopharyngeal tumor. Other suspicious symptoms include oral pain, nonhealing ulcerative lesions, dysphagia, odynophagia, hemoptysis, epistaxis, headaches, nonhealing dental infections, and a nonpainful neck mass. More advanced disease may also be associated with systemic symptoms such as anorexia and weight loss, fatigue, and even neurocognitive changes.
nasal congestion could indicate a nasopharyngeal tumor. Other suspicious symptoms include oral pain, nonhealing ulcerative lesions, dysphagia, odynophagia, hemoptysis, epistaxis, headaches, nonhealing dental infections, and a nonpainful neck mass. More advanced disease may also be associated with systemic symptoms such as anorexia and weight loss, fatigue, and even neurocognitive changes.
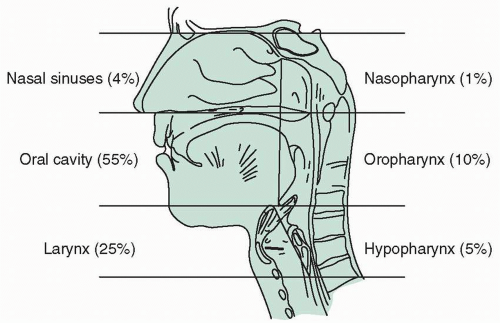 FIGURE 6.1 Anatomic divisions of the head and neck. Percentages indicate the relative frequencies of carcinoma in these regions. |
B. Evaluation
Patients who are ultimately diagnosed with HNC will often present initially to a primary care physician, a dentist, or directly to an otolaryngologist, so an awareness of the possibility of cancer is important on the part of these providers. A careful history may help point to the primary site and suggest involved structures. A thorough head and neck evaluation will include an assessment of the primary site and the extent of nodal disease. Neck masses need careful assessment and often will require either fine-needle aspiration (FNA) or a core-needle biopsy to determine cancer and to provide adequate tissue for additional studies such as immunohistochemistry for HPV. An endoscopic examination is usually performed on initial evaluation by an otolaryngologist to identify and/or confirm the primary site. Most patients with laryngeal or pharyngeal tumors will undergo direct laryngoscopy with biopsy to determine the extent of disease and to rule out a second primary tumor. Imaging studies are considered a standard component of the workup in patients with locally advanced disease. The purpose of radiographic studies is to clearly define the extent of local disease, to identify nodal spread, and to rule out metastatic disease or
a second primary tumor. Computed tomography (CT) scans, magnetic resonance imaging (MRI), and positron emission tomography (PET) scans may each contribute unique clinical information; it is therefore important to discuss the appropriate radiographic evaluation with the radiologist in order to optimize the staging workup and to provide clinicians with the information needed for treatment planning. A cohesive multidisciplinary team is essential to the proper evaluation and treatment planning for these patients.
a second primary tumor. Computed tomography (CT) scans, magnetic resonance imaging (MRI), and positron emission tomography (PET) scans may each contribute unique clinical information; it is therefore important to discuss the appropriate radiographic evaluation with the radiologist in order to optimize the staging workup and to provide clinicians with the information needed for treatment planning. A cohesive multidisciplinary team is essential to the proper evaluation and treatment planning for these patients.
The HNC team consists of members representing all facets of the patient’s care and needs. This includes otolaryngologists with extensive training and expertise in HNC surgery, medical oncologists, radiation oncologists with expertise in complex radiotherapy protocols, pathologists, radiologists and nuclear medicine radiologists, dieticians, speech pathologists, oral surgeons and oral medicine specialists, psycho-oncologists and social workers, nurse navigators, and research coordinators. It is essential that all members of this complex team work seamlessly; this is often best accomplished in a multidisciplinary clinic setting with patient case discussion at tumor board conferences attended by all of the aforementioned members.
C. Pathology
Over 80% of HNCs are squamous cell carcinomas, typically showing areas of keratinization; however, basaloid (“jigsaw pattern”) features are more characteristic of HPV-associated squamous cell cancers. The remainder is composed of adenocarcinoma, mucoepidermoid, and adenoid cystic histologies; these latter types are seen mostly in salivary glands.
D. Staging
Treatment of HNC is based on primary tumor location and stage. Once a tissue diagnosis of HNC is obtained, a workup is undertaken to determine the clinical and/or pathologic stage of the tumor. This includes endoscopic evaluation of the throat, hypopharynx and larynx, and possibly the bronchial and esophageal areas; CT/MRI/PET imaging modalities; and biochemical assessment with complete blood counts, liver and kidney functions, and thyroid status. Tissue sampling of suspect areas is critical not only for histology and immunohistochemical (IHC) analysis but also for staging. Staging is based on the tumor, node, and metastasis (TNM) system defined in the American Joint Committee on Cancer (AJCC) staging system.5
Approximately one-third of patients present with localized disease (stages I and II); half present with locoregional disease (stages III and IV with nodal metastases); only about 10% present with distant metastatic disease. Early-stage or localized disease is often treated with a single modality, surgery or radiation therapy with an 80% to 90% long-term (5 years) survival. Locoregional disease (stages III, IVA, and IVB) is treated with multimodality therapy including various combinations and sequences of surgery,
radiation therapy, and chemotherapy. Long-term survival for this group of patients is approximately 40%. Patients with recurrent disease can occasionally be “salvaged” with surgery (approximately 15% of patients); however, many of these patients as well as those with distant metastases are incurable and are usually managed with either palliative chemotherapy or supportive care.
radiation therapy, and chemotherapy. Long-term survival for this group of patients is approximately 40%. Patients with recurrent disease can occasionally be “salvaged” with surgery (approximately 15% of patients); however, many of these patients as well as those with distant metastases are incurable and are usually managed with either palliative chemotherapy or supportive care.
E. Treatment
1. Pretreatment assessment
Even though HNC is usually confined to the head and neck area, a complete history and physical examination is critical as these patients often have comorbidities that affect their treatment. Tobacco, alcohol, and other substance abuse need to be identified and discussed. Patients who continue to smoke during radiation therapy do poorly and have increased mucositis; therefore, a robust smoking cessation program is important. Other comorbidities such as cerebrovascular disease, cardiovascular disease, renal insufficiency, chronic obstructive pulmonary disease (COPD), and alcohol-related disorders are frequently present and need to be managed. An accurate medication history is important; antihypertensives, diuretics, and antihyperglycemic agents may need adjustment or even discontinuation during treatment. Nephrotoxic agents (drugs and contrast for imaging studies) can be harmful in the midst of dehydration. Depression and suicide are not uncommon in HNC patients, thus initial and ongoing screening for mood disorders is appropriate, and psychosocial oncology support is important. Social workers are helpful in dealing with patients who have poor support systems and lower socioeconomic status. It is also recommended that all patients undergo an upfront evaluation by oral health specialists to assess for any necessary dental extractions prior to radiotherapy as well as recommendations for oral health care during treatment, such as fluoride trays, rinses, and other preventive measures. Speech pathology assessment and evaluation before treatment can identify potential dysphagia risks and provide advice for swallowing mechanics to minimize aspiration risks and reduce long-term gastrostomy tube dependence. Dietary assessment and determination of nutritional support requirements such as gastrostomy tube placements are important. All chemotherapy agents may impair fertility either temporarily or permanently, so fertility preservation methods should be addressed where appropriate. Baseline assessment of bone marrow, liver, and renal function as well as audiograms may be indicated due to potential toxicities with chemotherapy agents, including cisplatin, 5-flourouracil, taxanes, and other drugs.
2. Localized disease
Node-negative HNC can be managed with surgery alone in most cases, especially for small (T1-T2) tumors that are easily
accessible. Radiation therapy is an acceptable alternative for patients who are poor surgical candidates. Chemotherapy has little role in this setting.
accessible. Radiation therapy is an acceptable alternative for patients who are poor surgical candidates. Chemotherapy has little role in this setting.
Surgery is performed with curative intent with the aim to remove all disease with clear surgical margins. Neck dissections, either ipsilaterally or bilaterally, are determined on the basis of the primary tumor size and/or location, but are not routine in T1-T2, N0 tumors. Transoral robotic surgery (TORS) is an emerging technique that can be utilized in certain situations. Reconstructive measures are rarely needed for these early-stage tumors, but microvascular tissue “flaps” are occasionally needed to rebuild structural defects depending on site of the primary tumor and extent of surgery necessary.
Radiation therapy as a single modality has evolved over the past four decades with the development of intensity-modulated radiation therapy (IMRT), which has been shown to be useful in reducing long-term toxicity in oropharyngeal cancer, paranasal sinus cancer, and NPC. The use of altered fractionation schema and the incorporation of brachytherapy implants and stereotactic body radiation therapy (SBRT) have also been developed. Three-dimensional planning and improved understanding of tissue tolerance and physics of radiation have improved the delivery of higher doses, as much as 70 to 74 Gy in many cases. Careful treatment planning with pretreatment CT or PET imaging in the treatment position improves tumor delivery and takes into account moving structures affected by swallowing, breathing, and postural changes).6,7 Dental extractions, prior to treatment, to remove any teeth in the treatment field reduce the risk of mandibular osteonecrosis, but can delay the start of radiation by 2 to 4 weeks. Hyperfractionated or accelerated radiation protocols have shown a 10% to 15% improved locoregional control in phase II trials and a trend toward improved disease-free survival and overall survival in phase III trials. Acute toxicity (mucositis) was worse, but, at 2 years follow-up, late toxicity (xerostomia) was no different. A simultaneous integrated boost (SIB) technique, which utilizes “dose painting” with higher doses to the gross sites of tumor and lower doses to subclinical disease, is commonly used with conventional (five fractions/week) and accelerated (six fractions/week) schedules.8,9
3. Locoregional disease
Locoregionally advanced (LRA) disease is defined as stages III to IVB disease of the head and neck. This includes newly diagnosed patients with a T3-T4 primary tumor, advanced or unresectable nodal disease, patients with recurrent/persistent disease, and/or patients with advanced disease who are unfit for surgery. Despite the disease being advanced, these patients can still be treated with curative intent. An exception would be if the
patient has previously received radiotherapy and is deemed surgically unresectable, treatment would then be directed toward palliation. As mentioned previously, treatment usually involves input from a specialized multidisciplinary team and can involve options such as surgery, chemotherapy, and/or radiotherapy. The majority of patients with LRA disease are treated with definitive concurrent chemoradiotherapy in efforts to preserve the function of involved organs. Functional organ preservation approaches may not be appropriate in all situations, and surgical resection may be preferred if the tumor has already destroyed organ function, especially with laryngeal cancer. Other treatment options can include upfront surgery with postoperative radiotherapy or chemoradiotherapy, induction chemotherapy followed by chemoradiation, or salvage surgery after chemoradiotherapy. The decision on which combination would be optimal is made keeping multiple factors in mind, specifically, the patient’s age, performance status, comorbidities, size/location of primary tumor, organ function, nodal status, and patient preferences. Other factors should be considered as well especially those affecting adherence, including patient motivation, psychosocial issues, family support, and travel distance.
patient has previously received radiotherapy and is deemed surgically unresectable, treatment would then be directed toward palliation. As mentioned previously, treatment usually involves input from a specialized multidisciplinary team and can involve options such as surgery, chemotherapy, and/or radiotherapy. The majority of patients with LRA disease are treated with definitive concurrent chemoradiotherapy in efforts to preserve the function of involved organs. Functional organ preservation approaches may not be appropriate in all situations, and surgical resection may be preferred if the tumor has already destroyed organ function, especially with laryngeal cancer. Other treatment options can include upfront surgery with postoperative radiotherapy or chemoradiotherapy, induction chemotherapy followed by chemoradiation, or salvage surgery after chemoradiotherapy. The decision on which combination would be optimal is made keeping multiple factors in mind, specifically, the patient’s age, performance status, comorbidities, size/location of primary tumor, organ function, nodal status, and patient preferences. Other factors should be considered as well especially those affecting adherence, including patient motivation, psychosocial issues, family support, and travel distance.
Select patients will undergo primary therapy with surgical resection depending on the size, location, and extent of local invasion. This depends on the experience and technology available at the specific treating institution. Areas that are more approachable, such as the oral cavity, are more amenable to surgical resection. Technological advances, such as TORS and TOLM (transoral laser microsurgery), now allow for increasing accessibility to the oropharynx, hypopharynx, and larynx permitting organ-preserving surgery. Postoperative radiation is often incorpora ted after surgery to eliminate residual disease in cases with pathology showing high-risk disease (e.g., close margins, perineural invasion, lymphovascular invasion, or multiple positive nodes/nodal sites). Consideration of chemoradiotherapy should be discussed with an experienced multidisciplinary team for patients with high-risk features. Studies show that chemoradiotherapy is warranted for evidence of extracapsular extension or positive margins after surgery showing improved locoregional control and disease-free survival compared to radiotherapy alone.10,11
HNCs commonly metastasize to the cervical lymph nodes. Metastases can be clinically occult, but once locoregionally advanced, the prognosis is markedly affected. If a surgical strategy is employed upfront, after resection of the primary tumor, the surgeon will consider either a selective or a comprehensive neck dissection depending on the amount of clinically evident disease in the neck. For patients with no, or limited, evidence of cervical lymph node extension, a selective neck dissection will be
considered. This method depends on the location of the primary tumor as to which levels of lymph nodes will be removed. Oftentimes for tumors of the oral cavity, a selective lymph node dissection will involve the lymph nodes above the omohyoid in levels I to III and occasionally the superior region of level IV. For tumors arising in the pharynx/larynx, dissections will often involve lymph node levels II to IV and occasionally VI when deemed appropriate. When disease in the neck is extensive, including bulky disease, multiple nodal regions, or bilateral neck disease, a comprehensive neck dissection is usually employed. This is a more extensive surgery involving the removal of lymph nodes in levels I to V, and the term encompasses either the removal or the sparing of nonnodal structures such as the sternocleidomastoid muscle, spinal accessory nerve, and jugular vein.
considered. This method depends on the location of the primary tumor as to which levels of lymph nodes will be removed. Oftentimes for tumors of the oral cavity, a selective lymph node dissection will involve the lymph nodes above the omohyoid in levels I to III and occasionally the superior region of level IV. For tumors arising in the pharynx/larynx, dissections will often involve lymph node levels II to IV and occasionally VI when deemed appropriate. When disease in the neck is extensive, including bulky disease, multiple nodal regions, or bilateral neck disease, a comprehensive neck dissection is usually employed. This is a more extensive surgery involving the removal of lymph nodes in levels I to V, and the term encompasses either the removal or the sparing of nonnodal structures such as the sternocleidomastoid muscle, spinal accessory nerve, and jugular vein.
In the majority of patients with LRA disease, surgery is not indicated. This decision is based on the location of the primary tumor, extent of local invasion, and/or nodal involvement. These patients will be treated with either concurrent chemoradiotherapy or induction chemotherapy followed by chemoradiotherapy with efforts to preserve organ function. Combined modality treatment with chemotherapy and radiation is based on results of multiple randomized trials and meta-analyses.12,13,14,15,16 This approach came into favor with the results of the Veterans Affairs Laryngeal Study Group proving that combined modality treatment with sequential chemotherapy and radiation was equivalent in overall survival as compared to surgical resection with postoperative radiation.12 The long-term follow-up of the pivotal RTOG 91-11 trial proved that concomitant high-dose cisplatin with radiotherapy was similar in efficacy for laryngeal-free survival as compared to sequential chemotherapy followed by radiation. In this case, concurrent chemoradiation did show an improvement in locoregional control and larynx preservation, making this the new standard of care for LRA disease.13,14,15 Carboplatin is not as directly cytotoxic to tumor cells as cisplatin, but its effectiveness as a radiosensitizer is still in question. One trial suggests that carboplatin is not as effective as high-dose cisplatin with radiotherapy, but others argue that weekly carboplatin with radiation may be a reasonable option for patients with underlying renal dysfunction.17,18 Carboplatin has also been studied in combination with other agents such as 5-fluorouracil and paclitaxel. Some patients may not be able to tolerate platinum-based chemotherapy in general due to multiple factors. This group of patients may benefit from the addition of cetuximab, a monoclonal antibody (MoAb) inhibiting the epidermal growth factor receptor, in combination with radiotherapy as compared to radiation alone.19 Elderly or poorly functioning patients may consider radiation alone for palliation of symptoms.
Induction chemotherapy is still quite controversial as to its efficacy when compared to concurrent chemoradiotherapy. The TAX 323, TAX 324, and GORTEC trials established TPF (docetaxel (Taxotere), cisplatin, 5-flourouracil) as a more active regimen when compared to PF (cisplatin, 5-fluorouracil) as induction therapy prior to concurrent chemoradiotherapy; significantly improving survival, local control, and laryngeal preservation.18,20,21 However, these trials did not compare induction chemotherapy followed by chemoradiation versus chemoradiation alone. Trials that have evaluated this have shown mixed results.16,21,22,23,24,25,26,27 A recent phase II Italian study favored sequential therapy with higher complete response rate and better progression-free survival, but still no difference in overall survival.25 This led to a phase III trial with a 2 × 2 design comparing induction therapy with TPF versus no induction therapy followed by concurrent chemoradiation with either cisplatin-fluorouracil or cetuximab. At a follow-up of 33 months, the induction regimen showed statistically better progression-free survival and overall survival.28 This is a significant result as this is the first randomized trial to show improvement in overall survival; however, the 2 × 2 design of the trial makes extrapolation to clinical practice controversial at present and further follow-up as well as additional phase III trials will be needed to confirm. Induction therapy is still useful in a select group of healthy individuals at high risk of both locoregional recurrence and distant metastatic disease, specifically the subgroup of patients with bulky tumors/lymph nodes and low level nodal disease. Induction chemotherapy does come with the risk of increased toxicity leaving up to 25% of patients unable to finish full sequential therapy with combined chemoradiation, significantly increasing the risk of local relapse. Therefore, this question still remains unanswered and requires further evaluation for a definitive solution.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree