Gauri R. Varadhachary, Renato Lenzi, Martin N. Raber and James L. Abbruzzese • Carcinoma of unknown primary is a diverse group of heterogeneous cancers and accounts for about 2% to 4% of all cancers. • No universal agreement exists on the extent of evaluation to search for a primary cancer. • “Adequate,” early biopsy of a metastatic site is recommended to establish the diagnosis and help direct further workup. • Basic evaluation includes the following: • Comprehensive history and physical examination (including breast and pelvic examinations in women and testis and prostate examinations in men). • Routine laboratory tests, chest-abdominal-pelvic computed tomographic scan, and mammography in women. • Directed invasive tests based on symptomatology and pathological evaluation of the tumor tissue. • Judicious pathological assessment of the metastatic tumor sample including directed immunohistochemical markers. Additional molecular markers that have a therapeutic intent are based on clinicopathological evaluation (including KRAS mutational status, Her2 (ERBB2) expression, and epidermal growth factor receptor (EGFR) mutation studies). • The diagnostic utility of positron emission tomography (PET) is poorly defined; it is beneficial in selected patients. • The role of tissue of origin molecular profiling assays continues to evolve; these tests are beneficial in selected patients. • Empiric combination cytotoxic therapy and the “one treatment fits all” approach are no longer emphasized. • Where possible, individualized therapy for the metastatic cancer “profile” is based on detailed clinicopathological evaluation. • When pathological evaluation falls short (large differential), empiric platinum-based combination therapies are usually selected. • Taxane, gemcitabine, fluoropyrimidine + platinum doublet is a common “broad spectrum” first-line regimen in good performance status patients. • Additional lines of therapy depend on performance status, pathological “profile,” and response to first-line therapy. Carcinoma of unknown primary (CUP) is a diverse group of cancers with a poor prognosis. Despite the increasing array of sophisticated diagnostic tools available to establish the diagnosis of human neoplasia, oncologists have struggled to understand a subset of patients with metastatic cancer in whom detailed investigations fail to identify a primary anatomic site. The reported incidence of CUP varies with the practice setting and the definition used, but averages 2% to 4% of all patients who are diagnosed with cancer.1 Because identification of the primary lesion forms the basis for predicting the expected behavior and assigning appropriate therapy for malignant disease, the absence of a defined primary carcinoma poses a major challenge. The inability to identify a primary carcinoma also generates anxiety for the patient, who may feel that the physician’s evaluation has been inadequate or that the prognosis would be improved if a primary site could be established. The management of CUP has been evolving, and recently developed sophisticated diagnostic modalities and novel therapies present both an opportunity and a challenge. As suggested by the range of incidence statistics, the definition of CUP has not been standardized, varying in published reports with regard to the extent of evaluation required to accept the diagnosis. For the purpose of this chapter, we define patients with CUP as having a biopsy-proven malignancy for which the anatomic origin remains unidentified after history and physical examination (including breast palpation and pelvic examination in women and testicular and prostate examination in men), laboratory studies including liver and renal function tests; hemogram; chest x-ray; computed tomography (CT) of the chest, abdomen, and pelvis; and mammography in women and measurement of prostate-specific antigen (PSA) in men.2 All positive findings on this initial evaluation are then investigated in detail. Depending on the clinical situation, additional studies might include directed invasive studies including upper endoscopy, colonoscopy, or bronchoscopy. To define the patient population further, most investigators have excluded from analysis instances in which soft tissue sarcoma or melanoma present without a definite primary site, because such presentations of these diseases have histology- and stage-specific therapy regardless of the primary. CUP clinical and research efforts concentrate on the vast majority of patients with common epithelial histologies such as adenocarcinoma, carcinoma, squamous carcinoma, and neuroendocrine carcinoma. Whether specific etiologic factors are relevant to CUP is not known. Although a history of cigarette smoking often can be elicited, the heterogeneity of CUP makes it unlikely that specific etiologic agents will be associated with this disease.3 The fact that numerous occult anatomic sites can give rise to carcinomas that present with only metastatic disease supports the possibility that specific interactions of genetic and environmental insults could give rise to genomic and biochemical changes that lead to the early development of the metastatic phenotype without the associated changes supporting local growth in the organ of origin. Although this concept is highly speculative, the hypothesis can be tested through analysis of available biomarkers such as oncogenes and tumor suppressor genes that have been characterized for cancers with known anatomic origins, such as lung, pancreatic, breast, and colorectal carcinomas. Either the absence of genetic changes typical for malignancies with established primary cancers or the presence of unusual variants of known genetic alterations would support this hypothesis. It is likely that as the genomic and proteomic characterization of malignancies is refined, fewer and fewer malignancies may be assigned to the CUP designation (and we are beginning to see that in this era of novel diagnostics). Whether the biology of CUP is fundamentally different from known primary carcinoma with systemic metastases remains controversial (Fig. 94-1). Nystrom and associates have argued that the distribution of metastatic sites in patients with CUP in whom the primary cancer is subsequently found is sufficiently different from known primary carcinoma to support the hypothesis that CUP is biologically unique.4 However, analysis of a series from the MD Anderson Cancer Center demonstrates few significant differences in the pattern of metastases or in overall survival for true CUP versus patients in whom the primary lesion was found.3 However, whether CUP metastases are genetically and phenotypically unique remains to be determined. The underlying cause for “occult” primary tumors is unknown. Previous investigators have speculated that the primary tumor may remain below the limits of clinical or radiographic detection or that it spontaneously regressed. It is possible that CUP falls along the continuum of cancer presentation in which the primary has been contained or eliminated by the immune system. Alternatively, CUP may represent a specific malignant event that results in an increase in metastatic spread or survival relative to the primary—with immunogenic or antiangiogenic factors that impede local growth.5 Aneuploidy, a well-recognized phenomenon occurring in 70% to 90% of solid tumors, is defined as a chromosome complement that is not a simple multiple of the haploid set. Increasing evidence indicates that for many carcinomas, such as breast, prostate, and colorectal cancers, a diploid DNA content is associated with a more favorable prognosis.6 Hedley and associates7 measured the cellular DNA content of tumor biopsy specimens of 152 patients with metastatic adenocarcinoma or undifferentiated carcinoma of unknown primary site to determine favorable subgroups. Aneuploidy was found in the specimens of 70% of the patients. There were no significant differences between men and women, and there was no obvious relationship of ploidy to the various patterns of metastatic involvement. The median survival of patients with diploid tumors was 4.2 months, versus 4.8 months for patients with aneuploid tumors. Of the 46 patients with diploid tumors, 9 (18%) survived for more than 2 years, compared with 10 (9%) of 106 patients with aneuploid tumors. These results indicate that the incidence of aneuploidy in this heterogeneous group of patients is similar to that reported for carcinomas with known primary tumors. However, in contrast to many of these tumor types in this study, metastatic adenocarcinomas of unknown primary origin that were diploid were not associated with a more favorable prognosis than those of known primary origin. The role of chromosomal abnormalities in CUP has been evaluated in several studies to better understand the biological characteristics of CUP. Abbruzzese and colleagues and Bell and coworkers identified common karyotypic changes in CUP.8,9 The karyotypes of 13 of 20 patients with CUP were determined, and in 12 of the analyzed cell lines, abnormalities were identified in the short arm of chromosome 1. The abnormalities detected included deletion of 1p, translocations, isochromosome 1q, and gene amplification. These findings were consistent with earlier descriptions of chromosome 1p abnormalities in advanced malignancy as described by Atkin29 and Mertens and associates.10 Motzer and associates used karyotyping to determine the frequency of specific abnormalities of chromosome 12 in patients with CUP.11,12 It was hypothesized that patients with undifferentiated carcinoma of unknown primary origin responding to cisplatin-based chemotherapy had unrecognized germ cell tumors and that isochromosome 12p, i(12p), a specific chromosomal marker characterizing germ cell tumors, could be used to identify such patients. Thirty percent of patients had an increased 12p copy number or deletion of the long arm of chromosome 12, which proved predictive of response, validating the hypothesis. Complete response to cisplatin-based therapy was achieved in patients with specific chromosomal aberrations associated with germ cell tumors, and objective responses were achieved in 75% of these patients, compared with 17% of patients without these aberrations. Summersgill and associates and Ilson and associates found similar associations for CUP patients with undifferentiated carcinoma.13,14 Thus for patients with undifferentiated carcinoma, i(12p) correlated with a good response to platinum-based chemotherapy, although lack of i(12p) may not exclude a small percentage of responses; i(12p) occurs in more than 80% of the germ cell tumors and only sparsely in a few other lesions (e.g., acute leukemia, embryonal rhabdomyosarcoma, and neuroepithelioma), and, therefore, determination of the presence or absence of i(12p) may assist in the diagnosis of extragonadal germ cell tumors in patients with CUP.15 These studies were conducted in the 1980s and 1990s; in the current era, pathological evaluation of cancers has significantly improved. Patients with extragonadal germ cell cancers are rarely seen in the CUP clinic and i(12p) testing is almost never needed to make a diagnosis or direct a therapeutic plan. The oncogenes ras, MYC, bcl-2, and her-2/neu are overexpressed in a variety of solid tumors. Pavlidis and associates found a high rate of overexpression of MYC (96%), ras (92%), and c-erbB2 (65%) in CUP cancers.16 However, investigators found that the overexpression of these genes was not related to histologic or clinical parameters or and did not have either diagnostic or prognostic value. Briasoulis and associates studied levels of bcl-2 expression in 40 patients with CUP (8% squamous, 36% adenocarcinoma, and 55.5% poorly differentiated carcinoma).17 Staining was evaluated based on intensity (+1 to +3) and the percentage of positive cells (1% to 100%). Expression of bcl-2 was observed in almost half of the tumors. This finding was not expected, because in most studies, bcl-2 had been found to be upregulated in premalignant lesions rather than in advanced malignancies and also had been associated with a less aggressive phenotype.18,19 In this study, the level of bcl-2 expression, by itself, had no prognostic value. When combined with a high level of expression of TP53, however, high expression of bcl-2 showed a trend toward a higher response to platinum-based chemotherapy. Hainsworth and associates stained 100 tumor specimens of poorly differentiated adenocarcinoma (PDA) or poorly differentiated carcinoma (PDC) of unknown primary site for Her-2 protein.20 The samples of 10 patients (11%) overexpressed Her-2. These investigators did not observe any major difference in the overall response rate to chemotherapy between the patients whose cancer overexpressed Her-2 and those who did not. Evaluation of the efficacy of trastuzumab in selected patients with CUP with Her-2 overexpression is warranted. Rashid and associates retrospectively evaluated 100 serial formalin-fixed, paraffin-embedded sections from patients with CUP with biopsies or resections done at MD Anderson Cancer Center.21 Seventy-six samples with adequate tissue were stained by immunohistochemistry for epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF)-A, Cox-2, c-erbB2, and KIT. Seventy-five percent of tumors stained for EGFR and 49% for VEGF-A. EGFR often was present with other markers, and was seen along with VEGF, c-erbB2, or Cox-2 in at least 30% of patients. Three triple-staining patterns of EGFR/c-erbB2/VEGF, EGFR/c-erbB2/Cox-2, and c-erbB2/Cox-2/VEGF were observed. Kaplan-Meier survival by intensity of staining for these proteins, however, failed to demonstrate significant associations. It is now known that a large number of gene deletions or allelic inactivation occur in most human cancers. Many of these genetic alterations result in the activation of oncogenes or in the loss of tumor suppressor genes and have been localized to specific chromosomes. To date, TP53 is the best known and most widely studied tumor suppressor gene. TP53 can control tumor development by arresting the cell cycle or initiating apoptosis of damaged cells. TP53 mutations are common and occur in about 55% of all human cancers.24–24 Briasoulis and associates evaluated TP53 expression using immunohistochemistry in 47 cases of CUP (4 squamous carcinoma, 17 adenocarcinoma, 26 PDC). Staining was evaluated based on intensity (+1 to +3) and percentage of positive cells (1% to 100%).25 More than 70% of the tumors expressed TP53; 53% expressed a high level and 47% expressed a low level of immunohistochemical staining. In this study, TP53 expression alone had no prognostic value. Bar-Eli and associates also investigated the frequency of TP53 mutations in a series of 15 CUP biopsies and 8 cell lines established from CUP.26 Mutations in the conserved regions of TP53 gene were analyzed by single-strand conformation polymorphism analysis of exons 5 to 9 and were verified by direct DNA sequencing of PCR products. The TP53 gene was mutated in 6 of 23 (26%) patients with CUP. Therefore, despite the fact that CUP tumors are often aneuploid, the frequency of TP53 mutation was relatively low in this study. This finding suggests that TP53 mutations may not play a major role in the development and progression of CUP. The discrepancy between the results of these two studies may be due to discordance between the results of immunohistochemical and genetic molecular methods in detecting the TP53 abnormalities as well as the “cancer types” and tissue studied. No research is available on metastasis-suppressor genes in CUP, which seem to play an important role in regulating the growth of disseminated cancer cells at secondary sites. Compelling evidence indicates that angiogenesis, as measured by microvessel density (MVD), correlates with the incidence of metastases in several solid tumors. Hillen and associates aimed to identify a specific biological role for angiogenesis in the metastatic phenotype of CUP by comparing MVD in liver metastasis of CUP with MVD in liver metastasis of colon and breast tumors.27 No difference was found between MVD in liver metastasis of CUP and known primary tumors. In CUP, as in other solid tumors, high MVD correlated with short survival in univariate and multivariate analyses. Karavasilis and associates investigated angiogenesis by assessing MVD and the tissue expression of VEGF and thrombospondin-1 (TSP-1).28 VEGF is a major stimulator of angiogenesis, and TSP-1 is an intrinsic angiogenic inhibitor. Paraffin-embedded archival material was evaluated from 81 patients diagnosed with CUP. Adenocarcinoma was the predominant histology found in 77% of cases, followed by undifferentiated carcinoma in 18% of cases and squamous cell carcinoma in 5% of cases evaluated. Tissue expression of CD34, VEGF, and TSP-1 was examined immunohistochemically by using specific monoclonal antibodies and was analyzed against clinicopathological data. The potential role of serum tumor markers in the evaluation and management of patients with CUP has been reviewed.29,30 As part of the evaluation of patients with CUP, five markers deserve special recognition. The beta subunit of human chorionic gonadotropin (β-hCG) has been classically associated with nonseminomatous germ cell tumors, and is useful both for diagnosis and during follow-up, often confirming the adequacy of therapy.31 Alpha-fetoprotein (AFP) also is useful for the evaluation of nonseminomatous germ cell tumors as well as hepatocellular carcinoma. Although many previous publications have suggested that the presence of elevated β-hCG or AFP identified patients with marked chemotherapy responsiveness and good survival (see following section on Poorly Differentiated and Undifferentiated Carcinoma), in one study the presence of abnormal plasma levels of AFP or β-hCG did not identify patients with better overall survival.32 Currently, β-hCG and AFP are indicated in patients with a midline tumor and a concern for extragonadal germ cell cancer. AFP is also indicated in patients with liver-predominant presentations and a risk for hepatocellular carcinoma. Measurement of prostate-specific antigen (PSA) is very useful in men with adenocarcinoma and predominantly skeletal metastases. Elevation of PSA can provide a confirmation of metastatic prostate cancer, but the physician should be wary of the occasional coexistence of early prostate cancer with a more aggressive synchronous second neoplasm. Serum measurements of PSA commonly should be coupled with immunohistochemical staining for PSA in tumor tissue, because rare patients have been reported with metastatic cancer and clinical features atypical for metastatic prostate cancer.33,34 Most tumor markers, including carcinoembryonic antigen (CEA), CA-125, CA 19-9, and CA 15-3, are not specific and are thus not helpful in determining the site of the primary tumor.35,36 Similarly, in our view, cytogenetic analysis is not helpful now that immunohistochemical tests are available. In a study by Motzer and colleagues, 40 patients with poorly differentiated carcinoma and CUP underwent cytogenetic analysis. Seventeen patients (42%) were diagnosed by genetic analysis, including 12 (30%) with cytogenetic changes characteristically seen in germ cell tumors (e.g., isochromosome 12p-i [12p], increased 12p copy number, or deletion of the long arm of chromosome 12).37 Pantou and colleagues studied 20 CUP samples, and in 5 patients (4 with lymphoma and 1 with Ewing sarcoma) cytogenetics aided in the diagnosis of the primary tumor. The other samples had multiple complex cytogenetic patterns.38 Unfortunately, these studies are obsolete in the current era of sophisticated immunohistochemical markers. The initial pathological assessment of the biopsy specimen is by light microscopic examination of paraffin sections stained with hematoxylin and eosin. Based on established cytologic criteria, in the presence of an adequate tissue sample, the pathologist usually can classify the tumor into broad groups such as carcinoma, sarcoma, or lymphoma.39 Additionally, many carcinomas will be immediately recognized as manifesting at least some glandular differentiation (adenocarcinoma). When glandular differentiation is scarce or absent, patients with CUP often are diagnosed with poorly differentiated carcinoma or undifferentiated carcinoma. Other specimens will lack any cytologic distinguishing features, in which case a diagnosis of an undifferentiated malignancy is reported. On light microscopy, about 60% of the cases are reported as adenocarcinoma and 5% as squamous carcinoma; in 35% of cases, light microscopy is not very helpful and poorly differentiated adenocarcinoma, poorly differentiated carcinoma, or poorly differentiated neoplasm is then reported. Immunohistochemical markers play a significant role in the diagnosis and workup of CUP (Table 94-1). They help define tumor lineage by using peroxidase-labeled antibody against specific tumor antigens. Direct discussions between the pathologist and clinician are critical to ensure the most accurate pathological characterization possible. Random use of large numbers of tissue markers is rarely helpful for establishing a diagnosis or planning therapy. The role of antibodies against AFP, β-hCG, PSA, and some other markers is well established. More recently, organ-specific stains are gaining more importance as markers for the identification of the origin of the carcinoma.40–46 Table 94-1 Commonly Used Immunoperoxidase Stains to Assist in the Differential Diagnosis of CUP Cancers Cytokeratin 20 (CK20) is a low-molecular-weight cytokeratin, which is expressed in normal glands as well as tumors of the GI epithelium, urothelium, and Merkel cell. Cytokeratin 7 (CK7) is found in tumors of the lung, ovary, endometrium, breast, pancreaticobiliary and upper GI tract cancers. TTF-1 is a nuclear protein that plays a role in transcriptional activation during embryogenesis in the thyroid, diencephalon, and respiratory epithelium. TTF-1 staining is typically positive for lung and thyroid cancers. In 2002, Roh and associates described the use of TTF-1 and CK20 in identifying the origin of metastatic carcinomas of cervical lymph nodes.40 They stained 68 specimens with TTF-1 and CK20. The primary sites were lung (29 cases), stomach (13 cases), colorectum (3 cases), and other sites (23 cases). TTF-1 expression was detected in 69% of metastatic lung carcinomas and in none of the GI carcinomas. CK20 expression was detected in 69% of GI tumors and in none of the metastatic lung carcinomas. Jang and associates looked at the use of these markers in identifying the origin of malignant effusions.41 The primary sites of the tumors examined were lung (16 cases), ovary (15 cases), stomach (9 cases), colon (8 cases), and breast (8 cases). The lung adenocarcinomas showed TTF-1 positivity in 81% of the cases (13 of 16), but all of the nonpulmonary adenocarcinomas lacked TTF-1 staining. The CK7−/CK20+ immunophenotype was seen in 63% of colonic adenocarcinomas and in none of the lung, breast, or ovary tumors. The CK7+/CK20− staining was observed in 100% of lung, 88% of breast, and 87% of cancers that originated from the ovary. They concluded that TTF-1 immunostaining was useful in differentiating between pulmonary and nonpulmonary origin of adenocarcinoma in malignant effusions. The combination of CK7−/CK20+ staining is useful in identifying colon adenocarcinoma. In 2000, Rubin and associates looked at the role of CK7 and CK20 in determining the origin of metastatic carcinoma of unknown primary site.42 The nuclear CDX-2 transcription factor, which is the product of a homeobox gene necessary for intestinal organogenesis, is expressed in normal colonic epithelia and most colorectal adenocarcinomas, and often used to aid with the diagnosis of GI adenocarcinomas.43 Some data suggest the role of cytokeratin 5/6 as a marker for squamous cell carcinoma in poorly differentiated tumors if mesothelioma is ruled out. Based on published studies, and with the addition of markers, a simple algorithm can be described (Fig. 94-2) for providing clinicians some guidance on using these immunohistochemical markers to identify the site of origin.
Carcinoma of Unknown Primary
Introduction
Etiology and Epidemiology
Biological Considerations
Aneuploidy
Chromosome Abnormalities
Oncogenes
Tumor Suppressor Genes
Microvessel Density
Patient Evaluation
Laboratory Studies and Serum Tumor Markers
Pathological Evaluation
General Considerations
Light Microscopy
Immunohistochemistry
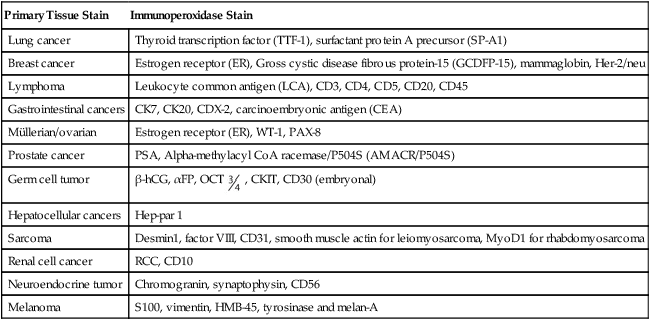
Primary Tissue Stain
Immunoperoxidase Stain
Lung cancer
Thyroid transcription factor (TTF-1), surfactant protein A precursor (SP-A1)
Breast cancer
Estrogen receptor (ER), Gross cystic disease fibrous protein-15 (GCDFP-15), mammaglobin, Her-2/neu
Lymphoma
Leukocyte common antigen (LCA), CD3, CD4, CD5, CD20, CD45
Gastrointestinal cancers
CK7, CK20, CDX-2, carcinoembryonic antigen (CEA)
Müllerian/ovarian
Estrogen receptor (ER), WT-1, PAX-8
Prostate cancer
PSA, Alpha-methylacyl CoA racemase/P504S (AMACR/P504S)
Germ cell tumor
β-hCG, αFP, OCT  , CKIT, CD30 (embryonal)
, CKIT, CD30 (embryonal)
Hepatocellular cancers
Hep-par 1
Sarcoma
Desmin1, factor VIII, CD31, smooth muscle actin for leiomyosarcoma, MyoD1 for rhabdomyosarcoma
Renal cell cancer
RCC, CD10
Neuroendocrine tumor
Chromogranin, synaptophysin, CD56
Melanoma
S100, vimentin, HMB-45, tyrosinase and melan-A
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Carcinoma of Unknown Primary