70 Carbon Ion Radiotherapy
The use of charged particles in medical radiotherapy (RT) was first suggested in 1946 by physicist Robert Wilson1, a former student of Ernest Lawrence, Nobel Prize winner for developing the cyclotron at the University of California, Berkeley. Following his participation in the Manhattan project and having been disappointed at the use of atomic weapons, Wilson was anxious to propose something useful for the welfare of mankind in the medical literature. At about the same time, Cornelius Tobias, also a student of Ernest Lawrence, was encouraged by Lawrence to work in the field of medical physics. Tobias was instrumental in preparing for clinical use of charged-particle beams with a long and productive series of biophysical studies beginning in the early 1950s2,3 at the University of California Lawrence Berkeley National Laboratory (LBNL). The rationale for using charged-particle beams of carbon ions for RT is based on dose-distribution advantages with less multiple straggling and enhanced biologic effects at depth resulting from the nonhomogeneous distribution of increased energy deposition around stopping particle tracks in the Bragg ionization peak, allowing more dose to the tumor and sparing of the surrounding normal tissues.2
When Robert Wilson proposed the use of protons in 1946, he also noted that carbon ions might be a useful beam and perhaps superior to protons. During the three decades between Wilson’s proposal until 1977, when the first carbon ion patient was treated in phase I trials by Castro and colleagues4,5 at the LBNL, a critical expansion of knowledge in RT occurred. Significant therapeutic gains were made with the use of high-energy x-ray beams with resultant increase in tumor control and fewer undesirable side effects. Much was learned from the pioneers of RT about the clinical application of these megavoltage beams, providing the basis for using charged particles in cancer therapy.
John Lawrence, brother of Ernest Lawrence, and John Lawrence’s colleagues in the late 1950s and 1960s used plateau proton beams for treatment of pituitary tumors at LBNL, employing a precise patient positioner with side-to-side head rotation.6,7 However, spread Bragg peak charged-particle therapy of cancer was only made practical with the advent of computed tomography (CT) in the 1970s. CT allowed rapid, accurate determination of the beam path through varying tissue densities in a patient, and powerful computers also became available for rapid treatment planning calculations.
Relative Biologic Effectiveness
The concept of relative biologic effectiveness (RBE) arose from observations that ionizing particulate radiations can be several times more effective per unit dose in producing biologic effects than x-rays. RBE is defined as the ratio of absorbed doses of two radiations required to produce the same biologic effect. One extension of this concept has been the clinical use of the terminology gray-equivalent (GyE) dose. This is the dose of a particle modality that yields an equivalent biologic response to a dose of megavoltage x-ray or cobalt-60 gamma-rays. For 160- to 230-meV proton beams, the RBE is generally considered to be approximately 1.1 to 1.2, and for heavier particles (helium, carbon, neon, silicon), the RBE ranges from 1.2 to 4.5. RBE depends on dose fraction size as well as the type of tissue irradiated and the position on the depth-dose curve at which it is measured. No single RBE value is adequate to characterize the variable range of RBE values in the stopping carbon ion beam, whether delivered by passive or active scanning techniques. When different clinical situations are considered, the biologic advantages of carbon ions over photons and protons are expected to be most pronounced for tumors that have a low radiosensitivity when treated with photons and for situations in which the tumor is surrounded by radiosensitive normal tissues. Values for RBE can be as high as 4 for carbon ion RT and depend on many factors, which have to be addressed during treatment planning. Experiments conducted with fast neutrons and carbon ions have demonstrated that increasing the dose per fraction results in a decrease of the RBE of both the tumor and normal tissues. The tumor RBE did not decrease as rapidly as the normal tissue RBE.8,9 As a consequence, short-course hypofractionation schemes are likely to enhance the therapeutic ratio in carbon ion RT. At the National Institute of Radiological Sciences (NIRS) in Chiba, Japan, hypofractionated carbon ion RT has been systematically investigated and a significant reduction of overall treatment time has been accomplished for many tumors without increasing toxicity.10–12
Decreased Radioresistance of Tumor Cells to High-Let Radiation
Although factors other than hypoxia are important in tumor control, most tumors have significant numbers of hypoxic cells that are preferentially killed with high-LET irradiations. Whether high-LET RT is effective in treating a given disease depends on the relative values of the tumor and normal-tissue RBE, as well as on reduction in the OER. Recent work with radioresistant tumor cells under even aerobic conditions in vitro indicate that stopping carbon ions can overcome tumor resistance as a result of the over-expression of Bcl-2, an anti-apoptotic protein that is frequently over-expressed in 30% to 50% of tumors and is associated with radioresistance.13 Carbon ions have also been shown to increase the gene expression of another protein, SPHK1 (a kinase related to cell growth and control of proliferation), in human oral squamous cell carcinoma in a manner not seen with x-ray doses.14 Qualitative differences between low-LET and high-LET molecular mechanisms of cell killing such as these need further study. Gene expression profiles are being used to identify novel candidate genes as biomarkers in human normal tissues and tumor cells associated with carbon ion radiosensitivity.15,16
Accelerated Tumor Reoxygenation
Carbon ion irradiation has been shown experimentally to accelerate the reoxygenation of murine fibrosarcoma compared with x-ray irradiation.17 This suggests that high-LET radiations would be effective with shortened treatment durations compared with low-LET RT.
Possible Increased Risk of Carcinogenesis and Other Late Radiation Effects
In high-LET RT the cost-benefit analysis must include an awareness of enhanced late sequelae, including undesirable tissue fibrosis, vascular damage, central nervous system (CNS) toxicities, genomic instability, and secondary cancers. In the clinical trial at LNBL, relatively short-term follow-up (24-173 months, mean of 67 months) of neon-ion treated patients suggested a radiation-induced tumor rate of approximately 2%.18 There was no increase over conventional x-ray rates for the helium patients. Carbon ion late effects should be similar to or slightly increased over helium ion effects. In the laboratory, there is clear genetic variability in the susceptibility to carbon ion–induced mammary carcinoma among rat strains studied.19 Sprague-Dawley rats were more susceptible to carbon ion–induced mammary carcinomas in a mechanism without the common H-ras and Tp-53 mutations seen with low-LET radiation-induced tumors. Further basic investigations should explore these molecular differences further with powerful new genomic and proteomic tools.
Preliminary Clinical Studies at Lawrence Berkeley National Laboratory with Heavy Ions
Clinical studies with fractionated Bragg peak charged-particle therapy, including carbon ions, were carried out from 1975 to 1992 at LBNL20,21 in collaboration with the Medical Center at the University of California, San Francisco. Helium ions were used instead of protons at LBNL, as they were readily produced in the LBNL accelerators at about 232 meV/U, giving a range in tissue of approximately 26 cm. They deposit a small amount of high LET, which must be accounted for in treatment planning. Their clinical effects are similar to protons, with normal-tissue RBE values of 1.2 to 1.4 compared with megavoltage x-rays (except in CNS, in which the RBE is approximately 1.6). Approximately 700 patients were treated with helium ions, and demonstrated excellent tumor control in critical sites in the skull base, eye, head and neck, and paraspinal-sacral regions, similar to results with protons.22–26
Carbon ions were used for the first time in the treatment of human tumors at LBNL in 1977. These studies were preliminary in nature, aimed primarily at assessing toxicity and tumor effect. Interestingly, the first patient treated with carbon ions in a skin RBE comparison with 6-meV electron-beam RT lived for more than 10 years. The late skin irradiation RBE values obtained for carbon ions of approximately 2.5 to 2.7 in the middle of a 4-cm spread Bragg peak was in line with estimates made later in Japan and Germany. A small number of patients with advanced tumors were treated with carbon ions in phase I studies at LBNL in 1977 and 1978. Subsequently, neon ions were picked over carbon ions for extended study at LBNL largely because of their greater potential for high-LET advantage. From 1978 through 1992, 433 patients at LBNL were treated with neon ion RT, of whom 299 received at least 10 Gy (approximately 30 GyE) of neon ions. A small number of patients (approximately 20) received treatment with silicon ions, and two patients were treated with argon ions. The LBNL clinical studies showed that neon and carbon ions could be delivered safely, providing careful treatment planning and delivery occurred, with evidence of beneficial effect especially in slow-growing tumors.21,27–30,30a Late reactions were increased in CNS tissues and GI tissues with neon ion RT. Concerns over significant late effects in normal tissues31–33 halted further study of silicon and argon ions.
Concomitant with the development of accelerator-based methods to provide carbon ion beams has been the advancement in tumor and normal-tissue imaging and beam-delivery techniques, including beam scanning and three-dimensional (3-D) and four-dimensional (4-D) breathing-gated delivery to provide individually-tailored image-guided dose plans.26,34–37 New technologies are also being developed commercially to accelerate carbon ions in facilities having a much smaller footprint and energy requirement for operation.38
Clinical Results of Carbon Ion Radiation Therapy in Japan and Germany
Carbon ion RT became available for the first time in a medically dedicated facility in 1994 when the NIRS in Japan started the first extensive clinical trials with carbon ions. The second carbon ion therapy facility, which began clinical operation in 1997, was developed by Kraft and associates39,40 at the GSI in Germany. This work will be continued at a new clinical heavy ion medical facility at the University of Heidelberg. A third carbon ion therapy facility began patient treatments at the Hyogo Ion Beam Medical Center facility in Harima, Japan, in 2004.41 Additional medical proton-carbon ion therapy facilities are planned or under construction in Italy, France, Germany, Austria, the United States, and Japan.
Beam Delivery Techniques at NIRS and GSI
Beam delivery is performed with passive methods using modulators, collimators, and compensators at the NIRS. The advantage of passive beam delivery systems is that the treatment planning for this system is simple. This approach has already been applied in many clinical situations, including in the treatment of moving targets such as lung cancer and liver cancer. The major disadvantage is that a significant amount of the dose is also delivered along the entrance path that often includes nontarget normal tissue. As an alternative to passive beam delivery, active beam delivery using the raster scan method has been developed. At GSI, focused pencil beams produced in a Synchroton are deflected laterally by two magnetic dipoles while the energy of the incoming beam is varied during the treatment. Thus 3-D intensity-modulated carbon ion RT can be accomplished, and the dose distribution can be tailored optimally to any irregular tumor shape without adding passive absorbers, compensators, or collimators.42 The dose distribution can also be conformed to the proximal edge of the target volume, and normal tissue that resides along the entrance channel of the beam can thus be spared. For both passive and active beam delivery techniques, patient immobilization and its day-to-day reproducibility need to be consistent with the high spatial accuracy achievable with particle beams. Precision head and body immobilization systems, stereotactic target localization, and image guidance with pretreatment correction of even small patient set-up deviations are commonly used at modern particle therapy centers. Using passive beam delivery, interfractional and intrafractional movements are addressed by adding a safety margin to ensure that the clinical target volume (CTV) is fully covered during treatment. For active beam delivery, adding a safety margin around the CTV is not sufficient, because movement of the target during the scanning process can cause regions already irradiated to again move into the path of the beam, whereas regions that have not been irradiated can escape the path of the beam. Different strategies of tumor tracking are currently being explored.43–45 The use of an active beam delivery system also has implications in terms of the treatment planning procedure, which becomes more complex and requires biologic plan optimization. New computational modeling strategies to optimize the LET distribution over the tumor volume are under development for each type of beam delivery mode.46,47 The physical advantages of particle-beam therapy can only be properly exploited when it is possible to use multiple fields at the same level of complexity commonly used for modern photon therapy. Although gantries for proton therapy have been installed at several facilities, carbon ion RT is delivered with fixed-beam lines. The charged-particle therapy facility at Heidelberg University is the first to be equipped with a rotating gantry for proton and carbon ion RT. The optimal design of a modern particle therapy facility is still actively being discussed.
Uveal Melanoma
RT of uveal melanoma aims at local tumor control and eye retention. Although brachytherapy and proton RT are accepted function-preserving alternatives to surgery with 5-year local control rates in the range of 96% and eye retention rates between 75% and 92%,22,48–54 carbon ion RT has been investigated recently at the NIRS in a dose-escalation trial in large-size uveal melanoma. Data is preliminary because of the limited patient number and follow-up time. Between January 2001 and February 2006, 59 patients with locally advanced or unfavorably located choroidal melanoma were enrolled in a phase I and II trial investigating an optimal dose of carbon ion RT. The primary endpoint of the study was morbidity, and local control and overall survival were secondary endpoints. Local control, overall survival, and disease-free survival rates at 3 years were 97.4%, 88.2%, and 84.8%, respectively. Twenty-three patients (40%) developed neovascular glaucoma and three patients underwent enucleation.55 It was found that the volume of the ciliary body irradiated to more than 50 GyE and irradiation of the optic disc were independent risk factors for development of neovascular glaucoma after carbon ion RT in choroidal melanoma.56
Skull-Base Chordoma and Low-Grade Chondrosarcoma
Chordoma is a rare tumour with a slow growth pattern. Thirty-five percent of chordomas occur in the skull-base region, displaying a challenge for neurosurgeons as well as radiation oncologists. Complete resection is exceptional and local recurrences are common. The results after treatment of chordoma and low-grade chondrosarcoma with conventional photon RT and stereotactic photon RT have been poor, with prognosis ranging between 17% and 50% at 5 years.57–59 At the Massachusetts General Hospital, 375 patients with skull-base chordoma and chondrosarcoma have been treated with proton RT between 1975 and 1998. Local control rates at 5 and 10 years were reported to be 73% and 54% for chordoma and 98% for chondrosarcoma, respectively.60 Other proton centers report similar results.61–63 Currently, proton RT is considered the treatment of choice for patients with chordoma and low-grade chondrosarcoma of the skull base.
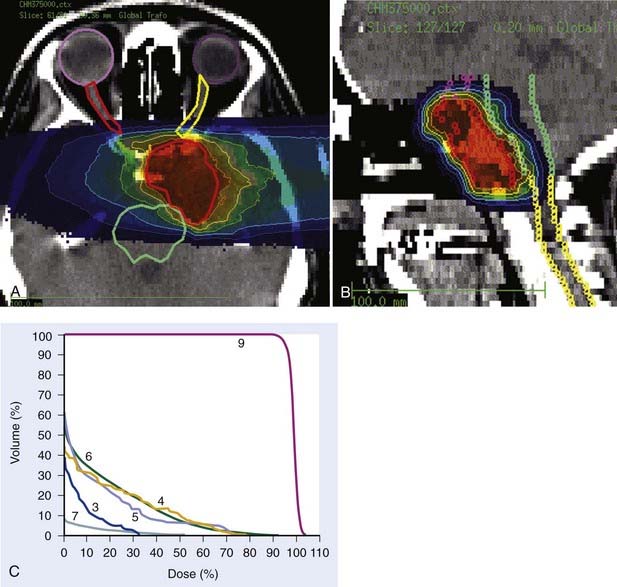
Carbon ion RT also offers biologic advantages in treatment of these tumors, particularly chordoma. Between 1997 and 2001, a clinical phase I and II study was carried out at GSI to investigate the feasibility and effectiveness in chordoma and chondrosarcoma of the skull base. Sixty-seven patients with chordoma (n = 44) and low-grade chondrosarcoma (n = 23) were enrolled in the study and received a full course of carbon ion RT with a median total tumor dose of 60 cobalt gray equivalent (CGE) (range 57 to 70 CGE/20 fractions/20 days). A typical carbon ion treatment plan of a patient with skull-base chordoma is shown in Fig. 70-1. Median follow-up was 32 months (range 3 to 66 months). Local control rates for chordoma and low-grade chondrosarcoma of the skull base were 74% and 87% at 4 years, respectively. Overall survival at 4 years was 86% and 100% for chordoma and chondrosarcoma, respectively. Acute toxicity was very mild. No patient developed acute skin reaction greater than the National Cancer Institute common toxicity criteria (NCI-CTC) grade 2. Only three patients developed grade 3 mucositis.64 A follow-up analysis in 2005 of this study included 96 patients with chordoma and 54 patients with chondrosarcoma. Cumulative local control and overall survival rates at 4 years were 89.8% and 98.2% for chondrosarcoma.65 Local control and overall survival rates at 5 years were 70% and 88.5% for chordoma of the skull base.66 Severe late toxicity was observed in less than 5% of all patients, and overall treatment time was significantly reduced to 3 weeks.
Carbon ion RT–induced toxicity to the temporal lobes was analyzed in 59 patients with chordoma and chondrosarcoma treated between 2002 and 2003 at GSI with carbon ion RT. Of these 59 patients, 10 developed circumscribed white-matter changes in the temporal lobes during their regular follow-up with magnetic resonance imaging. In most of these patients, the white-matter changes were of a temporary nature and diminished without treatment. Only two patients developed neurologic deficits. Analysis of the dose-volume histograms revealed that age and maximum dose, after subtracting the maximum dose volume of 1 ml, significantly influenced the risk for white-matter changes after carbon ion RT. The 5% and 50% probabilities for temporal-lobe injury after carbon ion RT were determined to be 68.8 and 87.3 GyE,67 consistent with highly conformal photon and proton irradiations.
When the local control rates in chordoma were analyzed together with the data available for proton RT, a dose-response relationship could be derived for chordoma of the skull base. The optimal dose is estimated to be between 75 and 85 GyE.66
The results of carbon ion RT at NIRS in 29 patients with chordoma and seven patients with chondrosarcoma were recently reported.12 The total dose was escalated from 48.0 to 60.8 GyE given in 16 fractions for 4 weeks. Overall local control was achieved in 74% of chordoma patients and in 100% of those with chondrosarcoma. With higher total doses, an improvement in tumor control was observed with no severe adverse reactions. For chordoma, the fractionation regimen of 60.8 GyE given in 16 fractions for 4 weeks yielded the best local control of 91% at 5 years, whereas local control was only 60% after 48.0 to 57.6 GyE. The overall 5-year survival rates were 88% in chordoma and 54% in chondrosarcoma. There were three deaths with chondrosarcoma, all with intercurrent disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree