Cancers of the Thorax
Feng-Ming (Spring) Kong
Jian-Yue Jin
Jeffrey D. Bradley
Mary K. Martel
Introduction
Thoracic tumors include lung cancer, thymomas, mesothelioma, esophageal cancer, and other less common tumors, such as lymphoma, germ cell tumor, and sarcoma. This chapter will cover only lung cancer, thymomas, and mesothelioma since the others are covered elsewhere in this book. Lung cancer is the most commonly diagnosed cancer worldwide (1.3 millions in 2002, 1.6 millions in 2008) (1,2). In 2009, there were an estimated 219,440 new cases and 159,390 deaths from lung cancer in the United States (3). Thymic tumors (4) and mesothelioma, on the other hand, are relatively uncommon (5). Radiation therapy (RT) plays a significant role in the management of thoracic tumors. The majority of these tumors require radiation therapy (RT) as a local measure for (i) definitive treatment of medical inoperable or surgically unresectable diseases; (ii) part of a multimodality regimen for locally advanced disease; (iii) adjuvant intent, needed prior to (neoadjuvant) or after surgery (adjuvant); or (iv) palliation of symptoms in patients with stage IV disease. This chapter reviews RT planning procedures, including steps before RT decision is made, such as diagnosis and staging workup, defining the role of RT; and steps after RT decision is made, such as defining dose and fractionation, simulation, defining target, dosimetric treatment planning, and RT techniques, for tumors of lung, thymus, and mesothelial cells. This chapter will focus on lung cancer, including both non-small-cell lung cancer (NSCLC) and small cell lung cancer (SCLC), and cover thymomas and mesothelioma, with each of the disease site divided into two subsections: procedures before and after the RT decision is made.
Lung Cancer
Lung cancer is the leading cause of cancer deaths in the United States—among both men and women. It claims more lives than colon, prostate, and breast cancer combined. Treatment for lung cancer is based on the type (NSCLC vs. SCLC), stage of tumor, and the patient’s general medical condition. Options include surgery, chemotherapy, and most frequently, a combined modality therapy. It is estimated that >60% of lung cancer cases require RT at least once, with about 45% receiving it as part of their initial treatment (6).
Diagnosis and Staging Workup
Correct diagnosis and staging is essential for determining treatment and providing prognostic information. The tumor, node, and metastasis (TNM) system (AJCC 2010) (Table 33.1) should be used for staging. For SCLC, the tumor is further grouped as limited and extensive stage. Limited-stage SCLC (LS-SCLC) defines tumors that are encompassed within a reasonable radiation port, which often requires that the tumors be confined to the hemithorax without malignancy pleural effusion. Staging workup should be sequential and logical in order to avoid unnecessary, expensive or invasive tests, and extensive staging is unnecessary for a clearly palliative treatment. The end points are to (i) establish a histology diagnosis; (ii) obtain precise anatomical, and if possible, pathological, staging of the patient; and (iii) assess resectability and operability or direct radiation treatment planning. The workup should follow three steps: (i) clinical history and physical examination, (ii) clinical image staging, and (iii) pathologic diagnosis and staging.
Clinical History and Physical
All patients with newly diagnosed lung cancer should undergo a clinical history and physical examination. Particular note should be made of the overall performance status of the patient and of any weight loss. These two factors have significant predictive value for survival (7,8). The physical examination should be directed toward signs and symptoms of primary and distant metastatic disease, including bone pain, adenopathy, hepatomegaly, neurological changes consistent with brain metastasis, and subcutaneous nodules. Any pleural effusion should undergo cytological evaluation. Blood tests, including hemoglobin, alkaline phosphatase, transaminases, and lactate dehydrogenase (LDH) should be performed to look for evidence of distant metastases, particularly for SCLC, where the LDH test is prognostic and should be included in the lab request (9).
Clinical Image Staging
Virtually all patients should undergo a computerized tomography (CT) scan of the chest and upper abdomen,
which should include the liver, upper abdomen, and adrenal glands (10). Intravenous contrast should be administrated unless the patient has renal insufficiency or known allergic reaction to the contrast material. The CT scan provides excellent anatomical detail, which allows the T-stage to be established, including the relationship to fissures, mediastinal structures, or the pleura and chest wall. CT may provide signs for the resectability of masses contiguous with the mediastinum; criteria for probable resectability of masses include: (i) a contact length of <3 cm with the mediastinum; (ii) <90 degrees contact with the aorta; and (iii) preserved mediastinal fat layer between the mass and mediastinal structures (11). CT is less reliable with regard to signs of unresectability (11). Modern contrast-enhanced CT is highly accurate in detecting lymph node (LN) enlargement, but the clinical applicability of LN enlargement for staging the mediastinum is poor, because small nodes may contain metastasis and large nodes may be benign (e.g., in the case of post-obstructive pneumonia). Current practice uses >10 mm of the short-axis diameter as the criterion for nodal metastasis. A pooled data set yielded a sensitivity of 57%, a specificity of 82%, a positive predictive value (PPV) of 56%, and a negative predictive value (NPV) of 83%, with marked heterogeneity across individual studies for CT (Table 33.2) (12,13). CT can assist in selecting the best procedure for sampling suspected LN regions.
which should include the liver, upper abdomen, and adrenal glands (10). Intravenous contrast should be administrated unless the patient has renal insufficiency or known allergic reaction to the contrast material. The CT scan provides excellent anatomical detail, which allows the T-stage to be established, including the relationship to fissures, mediastinal structures, or the pleura and chest wall. CT may provide signs for the resectability of masses contiguous with the mediastinum; criteria for probable resectability of masses include: (i) a contact length of <3 cm with the mediastinum; (ii) <90 degrees contact with the aorta; and (iii) preserved mediastinal fat layer between the mass and mediastinal structures (11). CT is less reliable with regard to signs of unresectability (11). Modern contrast-enhanced CT is highly accurate in detecting lymph node (LN) enlargement, but the clinical applicability of LN enlargement for staging the mediastinum is poor, because small nodes may contain metastasis and large nodes may be benign (e.g., in the case of post-obstructive pneumonia). Current practice uses >10 mm of the short-axis diameter as the criterion for nodal metastasis. A pooled data set yielded a sensitivity of 57%, a specificity of 82%, a positive predictive value (PPV) of 56%, and a negative predictive value (NPV) of 83%, with marked heterogeneity across individual studies for CT (Table 33.2) (12,13). CT can assist in selecting the best procedure for sampling suspected LN regions.
Table 33.1 American Joint Cancer Committee Staging System for Lung Cancer | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
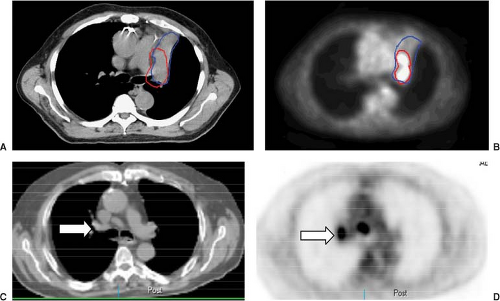
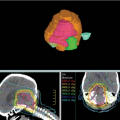
Positron emission tomography (PET) with 18 F-fluoro-2-deoxy-D-glucose (FDG-PET) is a standard component of current staging in patients with nonmetastatic NSCLC and SCLC. For NSCLC, PET is superior to CT in estimating T, N, and M disease. Although CT with its better spatial resolution remains the standard assessment of T-stage, FDG-PET may better characterize the primary tumor by differentiating tumor from the collapsed lung and add information in case of pleural involvement, where 89% sensitivity, 94% specificity, and 91% accuracy have been reported (14). Data on nodal staging with PET have been addressed in several meta-analyses (12,15,16) (Table 33.2), and convincingly demonstrate that PET is superior to CT in staging mediastinal LN. In general, PET has an excellent NPV for nodal disease, which may allow omission of invasive staging in the case of an absence of FDG-uptake in the mediastinum in patients with low risk factors. However, a number of granulomatous or other inflammatory diseases also exhibit increased FDG-uptake. Positive findings on PET should be pathologically verified when possible, particularly when the disease is relatively confined to limited areas. PET-CT is better than FDG-PET alone. FDG-PET is also more accurate than other imaging modalities for detecting extrathoracic metastatic disease (17). In at least two studies, FDG-PET revealed unsuspected distant metastases in 10% to 30% of cases (17,18). Figure 33.1 shows examples of PET in assessing the extent of the primary tumor and detecting early nodal disease.
The ability of PET in better defining tumor extent has a significant impact on target delineation in the treatment planning of NSCLC, which will be discussed in section on “PET Scan on Target Definition.” PET is also superior to CT in restaging patients with NSCLC, which could be performed after induction therapy before surgery, after completion of a definitive nonsurgical intervention, or at recurrence after surgical resection (19,20).
Interpretation of PET images could be improved by visual correlation with CT, due to better localization of PET abnormalities using the anatomical detail of CT. Fusion PET-CT scanners, obtaining the coregistration of functional-molecular and morphological-anatomical data, can further improve T, N, and M staging accuracy (19,21,22). FDG-PET-CT is also more accurate than other imaging studies for detecting extrathoracic metastatic disease (17).
Magnetic resonance imaging (MRI) is of limited value in staging lung cancer except in the case of intolerance to intravenous ionic contrast media, and in some special circumstances, such as assessment of the relationship of the tumor with large blood vessels, soft tissues, or vertebral body for patients with superior sulcus tumors (23).
Ultrasound of the liver, and brain CT or MRI are recommended for evaluation of distant diseases for high-risk patients (such as stage III), or patients with stage I/II with symptoms from the bone, liver, or brain, and in high-risk patients with nonspecific features of metastasis. These include unexplained anemia, unexplained weight loss of >5% of normal body weight in the previous 6 months, abnormal alkaline phosphatase or transaminase levels, and clinical suspicion of metastatic disease (24). For patients who have already had a PET scan, a bone scan is of less value, as PET is also reported to be more accurate in detecting bone metastasis. Based on a retrospective review of 257 cases, the accuracy of the PET and bone scan were 94% and 85% (p < 0.05), sensitivity values were 91% and 75%, and the specificity values were 96% and 95%, respectively (25).
Ultrasound of the liver, and brain CT or MRI are recommended for evaluation of distant diseases for high-risk patients (such as stage III), or patients with stage I/II with symptoms from the bone, liver, or brain, and in high-risk patients with nonspecific features of metastasis. These include unexplained anemia, unexplained weight loss of >5% of normal body weight in the previous 6 months, abnormal alkaline phosphatase or transaminase levels, and clinical suspicion of metastatic disease (24). For patients who have already had a PET scan, a bone scan is of less value, as PET is also reported to be more accurate in detecting bone metastasis. Based on a retrospective review of 257 cases, the accuracy of the PET and bone scan were 94% and 85% (p < 0.05), sensitivity values were 91% and 75%, and the specificity values were 96% and 95%, respectively (25).
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Pathologic Diagnosis and Staging
Histological diagnosis is mandatory and may be obtained using bronchoscopy, mediastinoscopy, and ultrasound-guided biopsy—bronchoscopy with biopsy is useful for T and N staging. Direct visualization and biopsy of the main bronchus and carina provide a 100% PPV for T4 disease. The same is true for transbronchial needle aspiration for mediastinal LN diseases. Endobronchial ultrasonography (EBUS) can visualize mediastinal LNs in the anterior, posterior, and inferior mediastinum at levels 2, 3, 4, and 7, as well as level 10 and 11 LNs. Esophageal ultrasonography (EUS) particularly visualizes LNs in the posterior part of levels 4 L, 3, 5, and 7, and in the inferior mediastinum at levels 8 and 9. The technique also complements mediastinoscopy and EBUS, for which cannot reach posterior subcarinal nodes or lower mediastinal nodes. Endoscopic ultrasonography may also help to exclude or confirm T4 disease in specific cases.
Mediastinoscopy remains the standard tool for invasive staging in patients without bulky mediastinal involvement. It has a good NPV and a 100% PPV (except for pathology misinterpretations) for nodal staging. Visual inspection at mediastinoscopy also distinguishes intra- from extra-nodal LN disease, and resectable from unresectable disease in difficult cases. Cervical mediastinoscopy—the most commonly used procedure—gives access to the pre-tracheal, right and left para-tracheal, and anterior subcarinal LN levels (levels 1, 2R, 4R, 2L, 4L, and 7), can be performed as an outpatient procedure and is reported to have very low mortality and morbidity in experienced hands (13). Patients with left upper lobe primary tumors may require additional evaluation of the subaortic area by left anterior mediastinotomy. Contraindications for mediastinoscopy are intolerance of general anesthesia, extreme kyphosis, and cutaneous tracheostomy. In some patients with central tumors, mediastinoscopy may also improve certainty of the T-stage, as it can prove unresectability due to the invasion of mediastinal central vascular structures. The sensitivity of cervical mediastinoscopy is reported as between 72% and 89%—on average 81% in a recent review with a pooled NPV of 91% (12). The results of the suboptimal sensitivity are partly explained by the fact that some LN stations (5, 6, 7 posterior, 8, 9) are not accessible by cervical mediastinoscopy. The recently implemented video-assisted procedure may further improve the accuracy. Left anterior mediastinotomy—the Chamberlain procedure—through the left para-sternal second intercostal space gives extrapleural access to level 5 and 6 LNs and assessment of resectability by palpating the tumor. Extended mediastinoscopy has been described as a technique to allow exploration of level 5 and 6 nodes via the cervical approach, by inserting the mediastinoscope through the cervical incision above the aortic arch. These techniques are more challenging, thus should be performed in left upper lobe tumors with suspicion of LN metastases in levels 5 and 6 based on CT and/or PET.
Video-assisted thoracic surgery (VATS) is also an important staging tool, which can allow biopsy of lesions in the contralateral lung or pleura. Similarly, LNs beyond the reach of conventional mediastinoscopy such as the inferior mediastinal LNs (levels 8 and 9) can be biopsied. LN stations 5 and 6 can be explored at left thoracoscopy, as an alternative to left anterior mediastinotomy. VATS is also an approach used in patients with advanced primary lesions in order to identify suitable patients for induction chemoradiation (ChemRT). As imaging studies do not always allow one to distinguish resectable T3 disease from unresectable T4 disease, an exploratory thoracotomy may still have to be performed.
Table 33.3 summarizes American Association of Clinical Oncology (ASCO) staging guidelines between 2003 and 1997. The staging workup recommendation has not been changed remarkably from 2003. Workup should be stopped at any point if distant metastasis is confirmed. For those with localized disease and medically fit for definitive treatment, additional tests are often needed to assess operability or pretreatment conditions. These include pulmonary function tests and quantitative ventilation perfusion tests. Pulmonary function tests are always required for surgery or radiation-based management. Quantitative ventilation/perfusion SPECT is sometimes indicated to help planning of surgical resection or RT in patients with marginal pulmonary functional reserve (26) Cardiac or other medical evaluations should also be performed as indicated. Unfortunately, there is no consensus even in the surgical world regarding surgical contraindications, and many patients still receive substandard surgical care (27). In general, patients with FEV1 >60% are considered to be operable without need of further testing. For those with FEV1 ranged 40% to 60%, maximum oxygen consumption should be >15 mL/kg. One should be advised that determination of operabilities or resectability is not based on certain numbers, rather it should be a decision of board-certified thoracic surgeons who perform lung cancer surgery as a predominant part of their practice.
The Role of RT in NSCLC
The choice of treatment for NSCLC is highly dependent on (i) stage and resectability of the tumor, and (ii) medical operability of the patient. The therapeutic modality with
the highest cure rates is surgical therapy by lobectomy or pneumonectomy (28,29,30). However, because of factors such as advanced stage, medical comorbidities, patient refusal, age, or other factors surgery may not be possible. It is estimated that only about 20% of patients initially presenting with lung cancer are eligible for definitive surgery (31,32).
the highest cure rates is surgical therapy by lobectomy or pneumonectomy (28,29,30). However, because of factors such as advanced stage, medical comorbidities, patient refusal, age, or other factors surgery may not be possible. It is estimated that only about 20% of patients initially presenting with lung cancer are eligible for definitive surgery (31,32).
Table 33.3 Staging Workup Guidelines from the American Association of Clinical Oncology for Patients with Advanced Non-small-Cell Lung Cancer (24) | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||
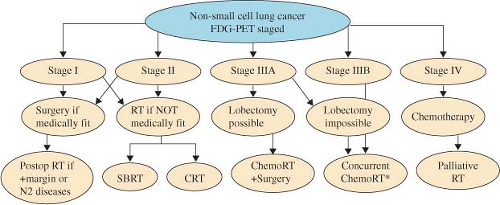
A flow chart with recommended therapeutic management for NSCLC is presented in Figure 33.2. RT plays following important roles: definitive RT for resectable NSCLC patients with medically inoperable or who refuse surgical treatment (stages I and II), adjuvant or neoadjuvant RT for resectable or potentially resectable patients (limited stage IIIA), ChemRT for unresectable diseases (stage III), and palliative RT for metastasis diseases (stage IV). Some patients with stage IV NSCLC could be treated aggressively when the distant disease burden is limited (such as solitary brain metastasis).
RT in Resectable NSCLC
Lobectomy is the treatment of choice for resectable NSCLC in patients who are medically fit for the procedure, with a 5-year survival rate after complete resection of 53%, 47%, 43%, 35%, and 26%, based on results of 68,463 patients of 20 countries treated from 1990 to 2000 from the IASLC database, for clinical stage T1a, T1b, T2a, T2b, and T3 disease, respectively (33). One must note that such procedure is associated with a significant morbidity
and mortality. A surgical pattern of care analysis reported a perioperative mortality rate of 5.2%, which ranged from 4.0% in patients who did not require a transfusion, to 12.7% in patients who underwent a transfusion (27). This mortality reflects the considerable comorbidity typically found in elderly patients.
and mortality. A surgical pattern of care analysis reported a perioperative mortality rate of 5.2%, which ranged from 4.0% in patients who did not require a transfusion, to 12.7% in patients who underwent a transfusion (27). This mortality reflects the considerable comorbidity typically found in elderly patients.
Table 33.4 Treatment Outcomes of SBRT and CRT (56) | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
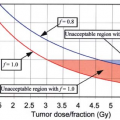
Definitive RT is the recommended treatment for patients with early stage NSCLC who are unfit for surgery and those who refuse surgical treatment. Radiation therapy improves survival in this group of patients (34). A median survival time of >30 months and a 5-year survival rate ±30% has been reported with fractionated RT alone (35,36). Local recurrence and distant metastasis are the main causes of failure, whereas regional nodal failure is uncommon (0%–7%) (7,37). A few selected studies from the use of conformal high dose RT reported 3-year survival rates around 20% to 30% (35,38,39,40,41,42). Stereotactic body radiotherapy (SBRT) is an emerging new technique and becomes the standard of care for T1 and T2 (<5 cm) N0, peripherally located NSCLC, with promising early results and limited acute toxicities (43,44,45,46,47,48,49,50,51,52,53,54,55). A meta-analysis of 30 studies (11 on 3DCRT, 11 on SBRT, 5 on proton, 3 on carbon ion) that reported 5-year overall survival rate in stage I inoperable NSCLC SBRT (42%) was significantly better than that for CRT (20%), similar to that of proton therapy (40%) and carbon-ion therapy (42%) (56). Tumor control outcome and results on radiation pneumonitis are shown in Table 33.4. RTOG 236, a recently published phase II trial from North America of 55 evaluable (44 patients with T1 tumors and 11 patients with T2 tumors) patients, reported an estimated 3-year primary tumor control rate of 97.6%—the 3-year primary tumor and
involved lobe (local) control rate was 90.6% and overall survival of 55.8% (54).
involved lobe (local) control rate was 90.6% and overall survival of 55.8% (54).
After surgical resection, cisplatin-based chemotherapy is recommended in patients with completely resected stage IB to III NSCLC. This is supported by results of multiple randomized trials. A recent meta-analysis based on collected and pooled individual patient data from the five largest randomized trials conducted by the Lung Adjuvant Cisplatin Evaluation (LACE) demonstrated that cisplatin-based adjuvant chemotherapy improved survival in patients with stage II or III cancer after surgical resection (57). Chemotherapy effect was higher in patients with better performance status (58). Another meta-analysis of 12 trials plus an individual patient meta-analysis (7,334 patients) demonstrated significant differences in favor of chemotherapy for overall survival in all seven subpopulation, with a relative benefit of 7% to 12% and an absolute benefit ranging from 2.5% to 4.1% (59). A very recent two meta-analysis (surgery plus chemotherapy vs. surgery alone, surgery plus RT, and chemotherapy vs. surgery plus RT) reported that the addition of adjuvant chemotherapy after surgery for patients with operable NSCLC improves survival, irrespective of whether chemotherapy was adjuvant to surgery alone or adjuvant to surgery plus radiotherapy (60).
Radiation therapy may increase local control and potentially improve survival in patients who have had resection for lung cancer. Postoperative radiation to the primary tumor bed should be given to patients with positive or suspicious margins, as the rate of local recurrence for this condition is over 50% (61,62). For nodal disease, the role of radiation remains to be controversial (63,64,65,66). An earlier meta-analysis of postoperative radiation therapy (PORT) concluded that adjuvant RT was detrimental for survival, especially for stage N0/N1 NSCLC (2000)(67). However, these conclusions have been widely debated, with critics focusing on the inadequate staging of patients, inclusion of early-stage patients, outdated radiation equipment and techniques, and inappropriate radiation dose-fractionation schemes (36). For patients with N2 disease, however, postoperative radiation reduced local relapses in the Lung Cancer Study Group (68) and both local and distant failure in the MRC trial (69). Recent studies using modern RT have failed to detect the detrimental effects (70,71), and a retrospective analysis by Sawyer et al. even suggested a survival benefit from PORT in N2 disease (72). Within the Surveillance, Epidemiology, and End Results (SEER) database of 7,456 patients treated with lobectomy or pneumonectomy, PORT was associated with a survival benefit in patients with N2 nodal disease (hazard ratio [HR] = 0.855; 95% CI, 0.762–0.959; p = 0.0077), though it was detrimental in N0/N1 disease (73). A recent subgroup analysis of Adjuvant Navelbine International Trialist Association (ANITA) suggested a positive effect of PORT in pN2 disease and a negative effect on pN1 disease when patients treated with or without adjuvant chemotherapy (64). In summary, adjuvant RT should be discouraged in patients with complete resected N0–1 NSCLC, and should be given in patients with positive or surpicious margins. Postoperative radiation with chemotherapy (after or concurrently) should be considered in patients with N2 disease. European Organization for Research and Treatment of Cancer (EORTC) trial to further evaluate the role of adjuvant RT in N2 disease is ongoing.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree