Cancers of the Head and Neck
Jonathan J. Beitler
Robert J. Amdur
William M. Mendenhall
Introduction
In the United States, the predominant risk factors contributing to the development of squamous cell cancers of the head and neck are related to lifestyle. Smoking and alcohol abuse are habits that disproportionately affect the poor. The least wealthy and the least educated patients are often ill-equipped to handle the impact of the cancer and the toxicities of treatment. Health care providers must address the disease as well as the socioeconomic needs of these patients. Therapy needs to be individualized, the patients educated, and the importance of both lifestyle change and good nutrition cannot be overemphasized. It has been estimated that if a patient continues to smoke during treatment, not only are the acute toxicities (and possibly the late complications) enhanced, but the response rates are lower (45% vs. 74%) as is 2-year survival (39% vs. 66%) (1).
In the Western world we have seen a tripling of the rate of oropharyngeal cancer, and this human papillomavirus (HPV)-related disease affects patients who are younger, and often neither tobacco smokers nor alcohol drinkers. From 1970 to 2002 the incidence of tonsil cancer increased 2.6-fold in men and 3.5-fold in women in a study from Stockholm (2). Using the national registry of Sweden, and looking at other smoking-related cancers such as lung and oral cavity cancers as reference, illustrates that the increase is not related to cigarette smoking (3). Looking at SEER data, the incidence of HPV-unrelated cancer was stable from 1973 to 1982 and significantly decreased from 1983 to 2004 (4). For individual HPV-related sites within the oropharynx, from 1973 to 2004 the annual percentage increase in base of tongue cancers and tonsil cancers was 1.27% and 0.6%, respectively, whereas the incidence of cancer was stable for other oropharyngeal sites. More recently, 85% of the tonsillar cancers biopsied between 2002 and 2007 in the county of Stockholm had HPV DNA recovered from their biopsy (5). The proportion of HPV-positive tonsil cancers nearly doubled each decade from 1970 until 2007 and from 2006 to 2007 the proportion of tonsil cancers with HPV DNA was 93%. With such a high proportion of these cancers being virally related, the role of prophylactic vaccination is being discussed (6).
The natural history of most head and neck cancers is an orderly and relatively predictable pattern of spread. Cancers tend to spread both locally and regionally, with regional disease being dependent on the anatomic location and extent of the primary tumor. As the affected neck nodes increase in size and exhibit extracapsular extension, the propensity for hematogenous dissemination increases; but distant metastases are a relatively uncommon pattern, and most patients presenting with head and neck cancer have the opportunity for cure.
Workup
After the history and physical examination, a biopsy, a detailed physical examination of the head and neck (including an endoscopic examination), and imaging are the work-up requirements. Computed tomography (CT) with intravenous contrast is the main imaging modality, although for nasopharyngeal cancer and advanced laryngeal cancers MRI will be more useful (7). Some patients with advanced disease of the head and neck may benefit from positron emission tomography (PET)/CT scanning to suggest the location of an occult primary, to provide functional data about lymphadenopathy and detect distant metastases. However, PET may underestimate the extent of local-regional disease that is apparent in the CT scan. Despite increasingly sophisticated imaging, the cancer-directed physical examination remains essential.
Immobilization
To assure setup reproducibility, optimum immobilization is essential. Often optimum immobilization is achieved by letting the patients obtain a comfortable position. Even without the changes in contour expected when a massive neck node resolves during treatment, through cancer- and treatment-related loss of muscle mass, patients may have difficulty maintaining certain positions. Consider the low-neck match. When matching two lateral field edges with an anterior field edge, inhomogeneity at the match line is an appropriate concern. In the past, particularly when primary disease was likely to extend inferior to the lateral fields, the patient was put in an uncomfortable treatment position to lower the shoulders so that the entire primary tumor could be encompassed by the lateral beams. Experience has suggested that achieving difficult treatment positions can be a
transient victory. Proper positioning may be maintained during the simulation and early in the treatment, but as muscles become weaker, reproducibility diminishes (8). In the era when many treatments for head and neck can be performed with intensity-modulating radiation therapy (IMRT), extensive lowering of the shoulders may be eliminated in many cases. As much immobilization as practical is endorsed and we use aquaplast casts to include the shoulders for immobilization. An important point to be noted is that the patient and not the cast is being treated, and marking the cast is a good first step in setting up the patient, but image-guided radiation therapy (IGRT) allows our therapists to concentrate on the bony anatomy expeditiously. Particularly in the head and neck, IGRT has been a big step forward in increasing treatment accuracy. We are exploring the role of weekly, cone-beam CT scanning during radiation.
transient victory. Proper positioning may be maintained during the simulation and early in the treatment, but as muscles become weaker, reproducibility diminishes (8). In the era when many treatments for head and neck can be performed with intensity-modulating radiation therapy (IMRT), extensive lowering of the shoulders may be eliminated in many cases. As much immobilization as practical is endorsed and we use aquaplast casts to include the shoulders for immobilization. An important point to be noted is that the patient and not the cast is being treated, and marking the cast is a good first step in setting up the patient, but image-guided radiation therapy (IGRT) allows our therapists to concentrate on the bony anatomy expeditiously. Particularly in the head and neck, IGRT has been a big step forward in increasing treatment accuracy. We are exploring the role of weekly, cone-beam CT scanning during radiation.
The bite block is commonly used for treating the oral tongue and the device needs to be used carefully. An effective tongue blade can push the tongue inferiorly and allow for mucosal sparing of the hard palate. The concern is that the bite blocks that most patients can tolerate are narrower than the tongue, and that the lateral aspects of the tongue could possibly extend above the tongue depressor, sometimes resulting in an insufficient margin. This is particularly important in the treatment of oral tongue cancers because the predominant site of involvement is the lateral aspect of the oral tongue.
General Treatment Guidelines
In the era of IMRT, the danger lies in specifying too small a target volume. This new technology affords us the opportunity to limit doses to the parotid glands and other important normal structures, but it should not be used in cases where it is likely to reduce the probability of cure. The guidelines for the initial treatment volume are to treat the gross and subclinical disease with expansion to a planning target volume (PTV) that makes sense for both the patients and the local expertise of the therapists. Typical PTV expansions range from 3 to 10 mm.
Intensity-Modulating Radiation Therapy—Practical Tips
Apart from early glottic cancers and situations where ipsilateral portals are indicated (e.g., lateralized tonsillar carcinoma), many patients with head and neck cancer may benefit from properly delivered IMRT if doses to uninvolved normal organs, such as the larynx and parotid(s), can be minimized. Although keeping the parotids below a median dose of 26 Gy does not absolutely prevent xerostomia, it reduces its severity. Patients should not have false expectations that they will have normal salivary flow after external radiation delivered with IMRT, but they should be assured that IMRT is a technique that may meaningfully improve their quality of life (9).
Treatment-planning CT is performed with 2- to 3-mm cuts; target volumes are identified on all cuts. Issues with matching the low supraclavicular field with the IMRT fields suggest the need to scan the entire supraclavicular region, so that a sensible treatment technique decision can be made. Treating the entire supraclavicular field within the IMRT fields eliminates the matching problem, but must be weighed against increasing the dose to the uninvolved larynx (10).
Intravenous contrast at the time of the CT simulation aids in delineating the primary tumor and the nodal disease, and enhances the visualization of the normal structures. Normal structures that need to be outlined include both parotid glands, the spinal cord, the brain stem, and, when relevant, the larynx, the submandibular glands, the thyroid glands, the lacrimal glands, the retina, the lenses, the optic nerves, the optic chiasm, the inner and middle ears, and the lips. When required by the RTOG, the brachial plexus needs to be outlined. Because few plans meet the brachial plexus constraints suggested by the RTOG protocols (11), do not underdose the CTV or GTV because of those constraints. Thus, outside of protocols, this contour is generally optional.
The parotid glands can be visualized by location and texture. The anatomic description by Netter (12) is as follows:
The parotid gland, the largest of the salivary glands, is roughly a three-sided wedge, which is fitted in below and in front of the external ear. The triangular superficial surface of the wedge is practically subcutaneous, with one side of the triangle almost as high as the zygomatic arch and the opposing angle at the level of the angle of the mandible. The anteromedial side of the wedge abuts against and overlaps the ramus of the mandible and the related masseter and internal pterygoid muscles. The posteromedial side of the wedge turns toward the external auditory canal, the mastoid process, and the sternocleidomastoid and digastric muscles.
The deep lobe of the parotid points toward, but does not reach, the carotid artery, wraps around the ramus of the mandible, and extends between the styloglossus and internal pterygoid muscles anteriorly and the posterior belly of the digastric and the stylohyoid muscles posteriorly. The CT appearance is somewhat akin to roughly ground glass.
The International Commission on Radiation Units (ICRU) Report 62 introduced the concepts of organ at risk volume (ORV) and the planning organ at risk volume (PRV). The spinal cord and other normal structures should be outlined and a 3-mm PRV margin should be added to the ORV for additional safety. At the University of Florida, all IMRT dose-volume histogram (DVH) displays are based on the PTVs and PRVs.
Conflicts between PRVs and PTVs are common and certain common sense rules have been developed. When dealing with overlaps of the spinal cord and the brain stem with the PTV, the PTV is more important than the PRV,
but the ORV is most important. Practically speaking, this means that the plan will spare the spinal cord or the brain stem when the 3-mm PTV expansion would overdose the spinal cord or the brain stem.
but the ORV is most important. Practically speaking, this means that the plan will spare the spinal cord or the brain stem when the 3-mm PTV expansion would overdose the spinal cord or the brain stem.
Table 28.1 University of Florida Dose Constraints for Head and Neck Intensity-Modulating Radiation Therapy | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
When the conflict is between the PTV and the parotid, submandibular, and lacrimal glands, and the mandible, oral cavity and the skin, the PTV is more important than the PRV or the ORV. Conflicts regarding the PTV and the visual structures need to be individualized. There are two main schools of thought on this: (1) Reduce the dose to part of the tumor to keep normal tissues below selected threshold doses, and (2) accept higher doses to the normal tissues to adequately treat the entire tumor. The latter approach is followed.
Tables 28.1 and 28.2 show normal tissue guidelines. Although IMRT may be used in patients with positive bilateral neck nodes, it is often difficult to adequately treat the neck and meaningfully spare one or both parotid glands.
Contouring Target Volumes
The gross primary and nodal disease as well as the high-risk subclinical disease sites are outlined by the physicians on the treatment-planning CT scan on the basis of physical and radiographic findings. At the University of Florida, these volumes are referred to as CTV 7200. Areas that have a 10% or greater risk of subclinical disease are contoured and labeled as CTV 4950. High-risk subclinical disease areas are contoured as a CTV 5400. The clinical target volumes (CTVs) are expanded by 3 mm with treatment-planning software to develop the PTVs, but special expansions for mobile structures, such as the oral tongue, may be necessary because of their potential for movement.
Table 28.2 Parotid and Submandibular Gland: Same With or Without Concomitant Chemotherapy | ||||||
|---|---|---|---|---|---|---|
|
Treatment of the Neck with Intensity-Modulating Radiation Therapy
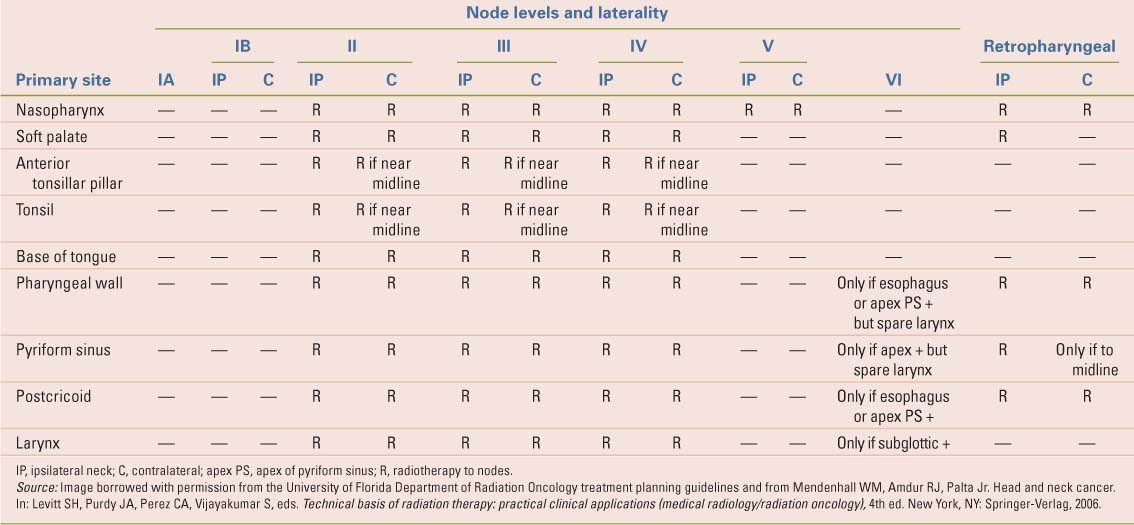
The likelihood of subclinical disease metastatic to the regional lymph nodes varies with the location and extent of the primary tumor. If clinically positive nodes are present, the risk of subclinical disease in the remaining cervical lymphatics also depends on the location and extent of the involved nodes. The definition of lymph node levels is depicted in Table 28.3. The clinically negative neck (or clinically negative regions of a node positive neck) are irradiated electively if the risk of subclinical disease exceeds 10%. The algorithm employed to determine which regions of the neck are to be treated is shown in Tables 28.4–28.6. Patients with well-lateralized T1-T2 N0-N2b tonsillar carcinomas are usually treated with ipsilateral field arrangements and the contralateral neck is not irradiated.
Table 28.3 Imaging-Based Nodal Classification | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
Table 28.4 N0 Bilaterally | |
|---|---|
|
Table 28.5 N1, N2 A, N2B, or Unilateral N3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 28.6 N2 C (Bilateral Neck Nodes) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Intensity-Modulating Radiation Therapy— Dose Guidelines
At the University of Florida, the concomitant boost schedule is used for most patients who are treated with definitive IMRT. All PTVs receive 54 Gy in 30 once-daily (q.d.) fractions. During the last 12 days, the CTV 7200 receives an additional 18 Gy in 12 twice-daily (b.i.d.) fractions with a 6-hour interfraction interval. Therefore, 72 Gy is delivered in 42 fractions over 30 treatment days when treatment is delivered 5 days a week. The initial plan delivers ∼49.5 Gy at 1.65 Gy/day in 30 fractions to intermediate-risk volumes. Plan optimization goals are to deliver the prescription dose to 95% or more of the PTV and to ensure that 99% of the PTV receives 93% or more of the prescription dosage to reduce the risk of injuring the visual apparatus.
Patients with lesions involving the skull base (e.g., nasopharynx; skin cancer with clinical perineural invasion) are treated with hyperfractionated IMRT to 74.4 Gy in 62 b.i.d. fractions to reduce the risk of injury to the visual apparatus.
Whereas RTOG 90–03 showed the superiority of hyperfractionated radiation over standard radiation when the patient was not receiving concurrent chemotherapy, RTOG 0129 added 100 mg/m2 of Cisplatinum on days 1 and 22 and randomized 721 eligible patients with locally advanced head and neck cancers to 72 Gy in 42 fractions delivered over 6 weeks versus 70 Gy in 35 (plus a third cycle of Cisplatinum). There was no difference in overall survival, local-regional failure, distant metastases, late toxicities, or feeding tube dependencies (13). One conclusion could be that in the setting of concurrent chemotherapy, hyperfractionation does not add benefit for these patients. Of course, in the era of IMRT, we are generally doing simultaneous in-field boosting as our high value targets are treated to higher daily doses than our intermediate or lower risk target volumes.
Oral Cavity
Most lesions of the oral cavity are treated surgically, but because the need for postoperative radiation therapy (RT) is common, the radiation oncologist should endeavor to examine the patient preoperatively. Despite the widespread use of postoperative RT, there are no randomized data that supports its use. Postoperative RT of the primary site is based on positive margins, bone invasion, invasion of the soft tissues of neck, perineural invasion, and endothelial-lined space invasion. Looser et al. reported that a margin of 5 mm or less is equivalent in impact to a positive margin (14). Indications for postoperative RT related to the neck include extracapsular extension and multiple positive nodes. In a multivariate analysis of patients treated with postoperative RT for oral cavity cancers, independent significant risk factors for local-regional failure were positive margins, vascular invasion, perineural invasion, extracapsular extension, and stage T3-T4 lesion (15). Only extracapsular extension and perineural invasion adversely impacted cause-specific survival. At the University of Florida, patients with negative margins receive 60 Gy in 30 fractions over 6 weeks. Patients with positive margins receive 66 Gy in 33 q.d. fractions or 74.4 Gy in 62 b.i.d. fractions with concomitant cisplatin (16). The larynx should be spared when treating the oral cavity and this can be done by placing the field junction between the primary fields and the low-neck field at the thyroid notch or 2 cm below the most inferior extent of the primary tumor. A tapered 1- to 2-cm wide midline block is placed in the anterior low-neck field and extends from the junction superiorly to the bottom of the cricoid cartilage inferiorly. IMRT can also be used to constrain the larynx within the context of the planning process.
Lips
For lip cancers, functional and cosmetic results are critical. Surgery can lead to microstomia as well as drooling and difficulty in eating due to lack of sensory and/or motor control. Therefore, RT is often preferred for oral commissure, upper lip, and larger lower lip cancers. Depending on the T-stage, the primary tumor (gross tumor volume [GTV]) is treated with a 1.5- to 2-cm margin, therefore defining the CTV.
Local treatment can be performed with brachytherapy, orthovoltage x-rays, or even photons, but most patients will receive definitive electron RT for the primary tumor (Fig. 28.1). The total RT dose usually varies from 60 to 70 Gy depending on the extent of the tumor and fractionation schedule. For electron RT, it is necessary to add an additional 1 cm to the lateral margins of the CTV for the PTV to account for beam constriction. One to 0.5 cm of bolus is used to ensure an adequate surface dose and the electron energy necessary to deliver 90% of the dose to a depth of 5 to 10 mm beneath the estimated deepest extent of the disease is selected. A lead shield is placed beneath the lip to reduce exit dose to the oral cavity and the mouth is kept open to move the uninvolved upper or lower lip out of the field.
As stated earlier, disease involving the oral commissure is best treated with definitive RT owing to the functional consequences of surgery. Local treatment fields should be generous and the regional nodes may require elective RT. Although patients with T1 N0 or T2 N0 lip cancers have a low regional failure rate of 4.8% (17), those with commissure involvement and/or T3/T4 primaries have significantly (18) higher rates of positive nodes and require regional treatment. Generally, the ipsilateral submandibular nodes should be the first echelon nodes followed by the level IIA nodes. The facial nodes are at relatively slight risk (19), but may be targeted. Other risk factors for nodal disease include recurrent disease, poor differentiation, and involvement of the “wet” mucosa.
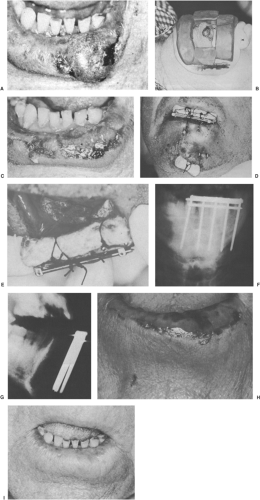 Figure 28.1. A 67-year-old man had T2 N0 squamous cell carcinoma of the lower lip. A: Lesion measured 3.0 × 2.0 × 1.5 cm. Radiation therapy was elected because of functional deficit likely to result from the excision of the large lesion. B: Lead mask, 2 mm thick, designed to outline portal. Lead putty was added to the shield to reduce transit irradiation to <1%. A separate lead shield covered with beeswax was inserted behind lower lip. The patient received 30 Gy in 2 weeks, 3 Gy per fraction, 250 kV (0.5 mm Cu). C: By the time of completion of 30 Gy, he had brisk mucositis of lip and approximately 60% to 70% regression of obvious tumor. D: Single-plane radium needle implant with double crossing. The pack was tied to the top of the bar to displace upper lip away from radiation, and the chin pack anchored the gingivolabial pack in place (see E). E: A gauze pack (arrows) sewn into gingivolabial gutter to displace radium from mandible, teeth, and gums. F and G: Anteroposterior and lateral views of implant. Implant added 35 Gy at 0.5 cm. H: 2.5 weeks after implantation. Note superficial ulceration. I: 22 months after treatment. No evidence of disease, and lip was completely healed. Nine-year follow-up revealed no evidence of disease. Source: From Million RR, Cassisi NJ, Mancuso AA. Oral cavity. In: Million RR, Cassisi NJ, eds. Management of head and neck cancer: a multidisciplinary approach, 2nd ed. Philadelphia, PA: JB Lippincott Co, 1994:321–400, Fig. 16.7, pp. 330–331.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|