Cancers of the Genitourinary Tract
Part A: Prostate
Daniel A. Hamstra
Michael E. Ray
Introduction
Prostate cancer is the most common malignancy in men in the United States with an estimated >190,000 new cases in 2009 and the second leading cause of prostate cancer death with >27,000 men presumed to die of prostate cancer in 2009 (1). The paradox in these numbers is the fact that <1 in 6 men diagnosed with prostate cancer will actually die from the disease. Therefore, the clinical evaluation and management of prostate cancer has changed dramatically over the last 10 years not only to improve treatment but also to better identify those who may not require treatment. The proven prognostic indicators including stage, grade, and pretreatment prostate-specific antigen (PSA) continue to be used for predicting outcomes with various therapies, although additional factors, such as PSA kinetics, are being increasingly considered.
Modern radiotherapy techniques for prostate cancer include external photon and particle beam treatments, as well as low dose rate (LDR) and high-dose rate (HDR) brachytherapy radioisotope implantation. Long-term follow-up of a number of trials utilizing external beam radiation therapy (EBRT) (2,3) have identified improvements in biochemical control of prostate cancer with only modest change in toxicity and patient reported quality of life as compared to conventional dose RT (4). These increases in RT dose without unreasonable increases in toxicity have occurred largely through the adoption of improved treatment techniques (4,5). Two-dimensional (2D) treatment planning techniques have at this time been essentially supplanted by the use of three-dimensional (3D) image sets based upon computed tomography (CT) and/or magnetic resonance imaging (MRI). Inverse planning and intensity modulated radiation therapy (IMRT) have become commonly used techniques that have further enabled the delivery of highly conformal treatment to targeted structures while minimizing radiation exposure to adjacent normal tissues (5). In addition, the use of image guidance (using such varied techniques as: ultrasound, implanted markers, cone beam CT, or electromagnetic guidance) to localize the prostate at the start of treatment and even during each delivered fraction has ushered in an era of image-guided radiation therapy (IGRT) (6).
Improvements in brachytherapy (both LDR and HDR) either alone or in conjunction with EBRT have also led to a number of ways to bring about intensification of local treatment with ionizing radiation. These may help in reducing the local failure component of prostate cancer; however, the distant metastatic rate, especially with large tumors and aggressive histology, is significant as well and it is still unclear how much an improvement locally intensified radiation provides on this front. In the past, prostate brachytherapy was done through an open surgical approach, and dosimetry was compromised by technical difficulty and lack of imaging techniques for accurate volume assessment and dose calculation. Today this procedure has been revolutionized by sophisticated preimplant or intraoperative treatment planning systems and the broad implementation of postimplant dosimetry. Generally, the implant is done through a template transperineally, with ultrasound for guidance. Iodine-125 (I-125), palladium-103 (Pd-103), and more recently cesium-131 (Cs-131) have been used for LDR permanent seed implants. There has also been increased use of automated iridium-192 (Ir-192) after loading units to deliver temporary HDR prostate implants.
Staging and Risk Stratification
Staging
Since the three most important prognostic indicators in prostate cancer are stage, grade, and pretreatment PSA, diagnostic evaluation is aimed at defining these factors. The clinical stage of the primary tumor is determined by digital rectal exam along with pertinent imaging studies. The American Joint Commission on Cancer (AJCC) clinical staging system for the primary tumor (T staging) is shown in Table 26.1 (7) The digital rectal exam was previously the main tool for detecting clinically localized prostate cancer, however, because of PSA screening and
heightened patient awareness, the majority of tumors are being diagnosed at earlier stages of disease and T1c (nonpalpable disease only identified due to an elevated PSA) now represents the most common clinical stage.
heightened patient awareness, the majority of tumors are being diagnosed at earlier stages of disease and T1c (nonpalpable disease only identified due to an elevated PSA) now represents the most common clinical stage.
Table 26.1 2010 American Joint Committee on Cancer (AJCC) Clinical Primary Tumor (T) Staging for Prostate Cancer (7th Edition) | ||
|---|---|---|
|
Unfortunately, digital rectal examination and transrectal ultrasound do a poor job of predicting disease extent at the time of biopsy or treatment. Therefore, there has been increasing interest in obtaining more accurate staging information using more sophisticated imaging modalities. Although often obtained, the utility of a staging CT scan is limited in most patients. CT scans accurately predict local tumor extent only 65% of the time (8). CT scanning is rarely able to detect involvement of metastatic lymph nodes (9), although the addition of fine needle aspiration may increase sensitivity (10). Staging CT scans are not recommended in patients with low-risk features such as early clinical stage, low PSA and Gleason score (GS) (11). Recent improvements in MRI have shown promise in enhancing clinical staging; although, in randomized trials MRI did not add value for prostate screening as compared to ultra-sound and DRE (12). Nevertheless, once cancer has been identified, endorectal coil MRI may have a role in predicting local extension of disease beyond the prostate capsule or into seminal vesicles with sensitivity and specificity of 65% and 100% in one study of low to intermediate risk patients undergoing prostatectomy (13). Newer MRI sequences such as diffusion weighted MRI or dynamic contrast MRI have also shown promise in limited evaluation although their role has not gained common acceptance (14). Since the most common distant metastatic site for prostate cancer is the skeleton, radioisotopic bone scan has been advocated as part of the staging workup. With the trend toward earlier diagnosis, the necessity of ordering this study has been questioned in many patients. In one study, the incidence of an abnormal bone scan was closely related to stage, GS and PSA, and was <1% among patients with clinical stage T2b or less, GS 7 or less, and PSA of 50 ng/mL or less (15). Therefore, this study need not be routinely ordered but rather applied only to patients at significant risk for disseminated disease.
Pathologic Evaluation
Given that PSA screening has led to more limited tumor stage coupled with the intrinsic limitations in clinical and radiological staging, histologic tumor grade and pretreatment PSA level have taken a profound role in patient evaluation and treatment selection. The Gleason system is based on the morphologic architecture of prostate cancer and is the method most often used for grading the aggressiveness of prostate cancer histologically (16). Based on the growth pattern and degree of differentiation, prostate cancer is graded on a scale from 1 (most differentiated) to 5 (least differentiated). The GS is the sum of the two most prevalent Gleason grades observed within a tissue sample and ranges from 2 through 10, with 2 the most indolent and 10 the most malignant. In conventional clinical practice, the GS is broken down into three main partitions: well differentiated (GS 2–6), moderately differentiated (Gleason 7), and poorly differentiated (Gleason 8–10). In addition, in contemporary clinical practice a GS of <6 is rarely encountered on needle biopsies due to a prevailing change in Gleason grading by pathologist which has led to a shift in GSs over time with higher GSs being more prevalent in the PSA era even as other clinical risk features have declined (17).
The serum PSA test is one of the most commonly ordered laboratory tests performed by thousands of clinical laboratories; however, there are a variety of manufacturers with commercially available serum PSA assays. The
values for serum PSA are reported in nanograms per milliliter with conventionally most labs reporting the lowest detectable value in the range of 0.1 to 0.2 ng/mL. However, there can be variation in reported thresholds and even levels between different laboratories using different assays. In addition, newer techniques have led to the development of ultra-sensitive or super-sensitive PSA tests that can detect PSA at one to two orders of magnitude lower level, which may be useful to detect recurrence (or predict a lower risk of recurrence) after a radical prostatectomy (RP). The utility of an “undetectable PSA” following RP using such novel PSA evaluations are still being determined (18).
values for serum PSA are reported in nanograms per milliliter with conventionally most labs reporting the lowest detectable value in the range of 0.1 to 0.2 ng/mL. However, there can be variation in reported thresholds and even levels between different laboratories using different assays. In addition, newer techniques have led to the development of ultra-sensitive or super-sensitive PSA tests that can detect PSA at one to two orders of magnitude lower level, which may be useful to detect recurrence (or predict a lower risk of recurrence) after a radical prostatectomy (RP). The utility of an “undetectable PSA” following RP using such novel PSA evaluations are still being determined (18).
Risk Stratification
Prostate cancer has a very heterogeneous natural history, as a result clinical factors are typically utilized to segment patients into risk groups that can be utilized to help describe the natural history of the disease as well as to select treatment regimens. The new AJCC Cancer Staging System (7th edition) includes a prognostic risk group system based upon T-stage, PSA, and GS (7) (Table 26.1); however, the most commonly utilized method for risk stratification at this time is the similar NCCN risk grouping (see Table 26.2) (19). The NCCN stratification system has been applied across large groups of patients treated with diverse treatment regiments and breaks patients into 5 categories based upon risk of recurrence: very low, low, intermediate, high, and very high (although most typically only low, intermediate, and high-risk groups have been utilized). However, it suffers in that there can be broad diversity of risk and as a result clinical outcome within the intermediate (20) and high-risk groups (21). Therefore, a number of additional means to risk stratify patients have been developed which are predominantly based upon pretreatment clinical factors. A large group of surgically treated patients has been used to devise look-up tables to predict extracapsular tumor extension, seminal vesicle involvement, and lymph node metastasis based on a combination of grade, stage, and pretreatment PSA level (11), and this data has been updated with a more contemporary cohort (22). Additional nomograms developed for predicting individualized outcomes after surgery or radiation therapy (RT) have also been developed (23,24). Indeed at last count well over 130 different algorithms or nomograms had been developed and reported upon to predict clinical outcome and other events (recurrence, metastasis, benefit from salvage radiation, etc.) (25). The exact role and discriminatory power of each of these tools are still being evaluated.
The clinical assessment of risk for local, regional, and distant disease is important for prediction of ultimate outcome and individualized counseling of patients regarding appropriate treatment strategies. Using the risk groups and/or nomograms described above, many practitioners utilize risk stratification schemes that group patients with clinically localized disease into low-, intermediate-, and high-risk categories. These groupings can be used to formulate appropriate treatment schemes for individual patients such as the one used at the University of Michigan for EBRT: as shown in Table 26.3. For radiation oncologists, it is important for the design of appropriate target volumes and selecting appropriate delivery methods and doses to be used for the administration of effective RT. In particular, the risk of seminal vesicle and pelvic lymph node involvement has a large impact on these decisions as does the use and duration of androgen deprivation therapy (ADT).
Table 26.2 Prostate Cancer Recurrence Risk Group By NCCN Criteria (2010 Version) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
Table 26.3 A Risk Stratification System for Clinically Localized Prostate Cancer with 3D Conformal or Intensity Modulated External Beam Radiation Therapy Treatment Guidelines utilized at the University of Michigan | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
Treatment Options
Watchful Waiting/Active Surveillance
For many men with prostate cancer the risk of clinical recurrence and/or death from prostate cancer may be small even without treatment. Therefore, the choice to observe without clinical intervention, particularly if patients are older or have an increased burden of co-morbid illness, remains a viable option and may even be the optimal treatment plan. The largest trial to address this was the Scandinavian Prostate Cancer Group-4 Randomized Trial (26). In this study men with clinically localized prostate cancer (25% T1 and 75% T2) were randomized to RP versus observation (watchful waiting) and at 12 years there was a 5% difference in the risk of death from prostate cancer in favor of those who underwent treatment (18% vs. 13%, p = 0.03). However, the 12-year rate of all cause mortality was not significantly different (22% vs. 18%, p = 0.09) There were, however, decreases in other clinically meaningful endpoints such as reduced metastasis with 19% of men in the treatment group and 26% of men in the watchful waiting group diagnosed with distant metastases at 12 years (p = 0.006). This trial pre-dated routine PSA screening and as such the majority of men had palpable disease on DRE and a median PSA >12. Therefore, the outcome with conservative therapy in the PSA era may have even smaller differences in clinical outcome which is supported by population-based analysis of prostate cancer outcomes in the USA (27).
An alternative to no active treatment (watchful waiting) for prostate cancer is to select patients for treatment based upon both initial clinical features as well as further clinical behavior in a process commonly referred to as “active surveillance.” A number of institutions have reported on outcome from such series with that from the
University of Toronto being the largest and most mature (28). In the Canadian experience men, on average 70 years of age and mostly with NCCN low-risk disease, were managed with an initial expectant approach, and definitive intervention was offered to those patients with a PSA doubling time <3 years, GS progression (to 4 + 3 or greater), or unequivocal clinical progression. Overall after a median follow-up of 7 years 70% of men remained on surveillance with a 10-year rate of death due to prostate cancer of 2.8% and the risk of death due to other cause being more than sixfold greater at 18.6%. A randomized study in North America and Europe is now applying this concept of active surveillance.
University of Toronto being the largest and most mature (28). In the Canadian experience men, on average 70 years of age and mostly with NCCN low-risk disease, were managed with an initial expectant approach, and definitive intervention was offered to those patients with a PSA doubling time <3 years, GS progression (to 4 + 3 or greater), or unequivocal clinical progression. Overall after a median follow-up of 7 years 70% of men remained on surveillance with a 10-year rate of death due to prostate cancer of 2.8% and the risk of death due to other cause being more than sixfold greater at 18.6%. A randomized study in North America and Europe is now applying this concept of active surveillance.
Radical Prostatectomy
Nevertheless, for patients who elect treatment for their prostate cancer the best curative treatment for clinically localized carcinoma of the prostate has been a contentious topic for many years. The advent of the nerve-sparing RP in the early 1980s, increasing chances for retaining sexual potency, as well as more recent refinements in surgical techniques such as laparoscopic or robotic RP, have ensured that RP remains a popular treatment option. Laparoscopic prostatectomy techniques have decreased acute surgical morbidity, with decreased blood loss, shorter hospital stays, and more rapid postoperative recovery (29). However, some concerns have been raised over possible increased risk of positive surgical margins and need for salvage therapy (30), particularly among surgeons new to the technique (31). In addition, patients with clinical evidence of extra capsular (T3) disease or high-risk features such as high GS or PSA may be at increased risk of residual disease after nerve-sparing surgery. Prostatectomy results in a substantial risk of impotence, and at least some degree of urinary incontinence is common (32,33). Nerve sparing techniques may decrease these rates although most patients still report significant sexual dysfunction even following nerve sparing RP (33,34,35). Although there have not been adequate direct comparisons, it appears that disease cure rates with RP are comparable to those seen with RT for low and intermediate risk disease (36). More recently there is also increasing emphasis on the use of RP for patients with clinically high-risk disease as well, which may necessitate a higher rate of utilization of postoperative RT (37).
External Beam Radiation Therapy
Definitive Therapy
EBRT is commonly utilized to treat men with clinically localized or locally advanced prostate cancer. EBRT alone may be applied to any clinical stage—T1 to T4—but is also often used with adjunctive anti-androgen therapy for selected higher-risk patients. We will address issues in regard to radiation dose, volume, technique below, and here will focus on the combination of ADT with RT and the role of RT after RP.
ADT
For high-risk prostate cancer, there is level I evidence from randomized trials supporting an overall survival advantage with the combination of ADT and RT. The question of ADT added to RT was first evaluated in a series of randomized trials which revealed that for patients with locally advanced (T3–T4) or high-grade prostate cancer (Gleason 8–10) ADT when added to conventional dose EBRT increased biochemical control and decreased risks of metastasis, death from prostate cancer, and all cause mortality (38,39,40,41,42). Therefore, for patients with the highest risk disease the use of ADT for between 28 and 36 months along with conventional dose EBRT appears better than either EBRT alone or EBRT delivered with shorter duration ADT (4–6 months) (40,42). For example, on RTOG 92-02 the addition of 24 months adjuvant ADT in addition to 4 months neo-adjuvant ADT resulted in a an absolute 16% increase in biochemical control at 10 years (p < 0.0001) along with improvements in local control (10% improvement, p < 0.0001), metastasis (8% improvement, p < 0.0001), and disease-specific survival (5% improvement, p = 0.004). Overall survival, however, was not increased for all patients, but was significantly improved for those with Gleason 8 to 10 disease (32% vs. 45%, p = 0.006). More recently it was also demonstrated that for node-negative men with high-risk prostate cancer the addition of conventional dose RT dramatically increased disease outcome when combined with long-term ADT as compared to long-term ADT delivered alone (43). There was an absolute risk reduction of 50% for biochemical failure at 10 years (75% vs. 26%, p < 0·0001) with the addition of RT. More importantly, the cumulative incidence at 10 years for prostate cancer-specific mortality was 24% in the ADT alone group and 12% in the ADT plus RT group (p < 0.0001). While the cumulative incidence for overall mortality was 39% in the endocrine alone group and 30% in the endocrine plus RT group (p = 0.004).
The role of ADT in intermediate risk disease has been addressed in two recent randomized trials which would suggest a benefit to shorter course anti-androgen therapy (4–6 months) when treated with conventional dose RT of < = 70 Gy (44,45). The role for ADT in patients treated with higher dose RT (particularly if intermediate risk) is an area of active evaluation at this time. Finally, there does not appear to be meaningful benefit with the addition of short-term ADT in either cancer-specific or overall survival in men with clinical low-risk disease by NCCN risk criteria even when treated with RT dose < 70 Gy (45).
Adjuvant or Salvage EBRT
EBRT is also commonly utilized as either adjuvant or salvage therapy in the post-prostatectomy setting for patients with clinical high-risk features or with a rising PSA following RP. For adjuvant therapy there is now level I evidence from randomized trials supporting a decline in biochemical failure, metastasis, and prostate cancer-specific death
with an improvement in overall survival with the use of adjuvant RT (ART) (46,47). The South West Oncology Group (SWOG) 8794 trial randomized men (stage pT3N0M0 disease or positive surgical margins) to observation or ART arms where the RT dose ranged from 60 to 64 Gy, with treatment portals including the prostatic fossa and paraprostatic tissues. With >12 years of follow-up the use of ART was associated with a 50% relative reduction in the risk of PSA recurrence in the ART group (p < 0.001). The use of ART yielded an absolute 10% improvement in metastasis-free survival at 10 years (71% vs. 61%, p = 0.016) and an 8% improvement in overall survival (66% vs. 74%, p = 0.023). Interestingly, the magnitude of benefit was similar for those with or without detectable PSA postoperatively although the utility of super-sensitive PSA remains to be determined (18). Two additional randomized trials support similar benefit in biochemical control of prostate cancer using ART although follow-up in these studies is insufficient to address more clinically meaningful end-points (48,49).
with an improvement in overall survival with the use of adjuvant RT (ART) (46,47). The South West Oncology Group (SWOG) 8794 trial randomized men (stage pT3N0M0 disease or positive surgical margins) to observation or ART arms where the RT dose ranged from 60 to 64 Gy, with treatment portals including the prostatic fossa and paraprostatic tissues. With >12 years of follow-up the use of ART was associated with a 50% relative reduction in the risk of PSA recurrence in the ART group (p < 0.001). The use of ART yielded an absolute 10% improvement in metastasis-free survival at 10 years (71% vs. 61%, p = 0.016) and an 8% improvement in overall survival (66% vs. 74%, p = 0.023). Interestingly, the magnitude of benefit was similar for those with or without detectable PSA postoperatively although the utility of super-sensitive PSA remains to be determined (18). Two additional randomized trials support similar benefit in biochemical control of prostate cancer using ART although follow-up in these studies is insufficient to address more clinically meaningful end-points (48,49).
The role of salvage RT for patients with a rising PSA after RP has not been evaluated in randomized trials (although certainly a significant portion of patients on both the SWOG and EORTC trials had detectable PSA at the time of irradiation and as such would be consistent with salvage therapy). In general approximately 30% to 40% of patients given salvage RT will have long-term disease control (50,51). Preoperative, pathologic, and other clinical features have been utilized to construct a nomogram predicting the likelihood of PSA control (51) which was subsequently validated in a large multi-institutional data set (50). In addition, others have presented data supporting an improvement in prostate cancer-specific survival with the use of salvage RT particularly in those with the most rapidly rising PSA (52). No studies have directly compared ART (delivered 10–12 w after surgery) due to the presence of high-risk pathologic features even with undetectable PSA to salvage RT delivered at the time of rising PSA. However, a number of retrospective reviews have suggested an improved rate of biochemical control using ART as compared to salvage RT (53,54). In addition, the role of ADT concurrent with either ART or salvage RT has not been clearly established with some favoring its use (55) with others finding no benefit to concurrent ADT with RT in the salvage setting (52). Nevertheless, in Great Britain it has been assumed that salvage RT is the standard of care and a study has been undertaken test if ART is superior to salvage RT, and at the same time to evaluate the role of ADT concurrent with RT using a four arm dual-randomized trial (the Radiotherapy and Androgen Deprivation in Combination After Local Surgery (RADICALS) trial) (56).
Brachytherapy
Interstitial brachytherapy is a popular alternative to EBRT for delivery of RT to the intact prostate. Potential advantages of brachytherapy over EBRT include the convenience of undergoing a 1-day outpatient procedure, the lack of organ motion that can confound EBRT planning and delivery, and the use of low-energy isotopes with rapid dose fall-off outside the prostate, potentially minimizing treatment-related morbidity. Most commonly, LDR permanent interstitial radioisotopic implants are performed using I-125, Pd-103, or more recently, Cs-131 seeds which are placed transperineally via needles inserted through a template. HDR brachytherapy can also be used by similar needle placement of catheters that are used for remote afterloading of a high activity Ir-192 source. Multi-institutional and long-term data suggest that brachytherapy performs comparably to surgery or EBRT for low-risk patients (57,58,59). Brachytherapy may also be combined with a conventional course of EBRT to 45 to 50.4 Gy, particularly for patients with adverse risk features (60).
Since the incidence of urethral stricture can be high after transurethral prostatic resection (TURP), implant is usually not recommended for stage T1a and T1b tumors. Other relative contraindications to brachytherapy include large prostate size, a prominent median lobe, or significant preexisting obstructive urinary symptoms, as these may be associated with technical difficulty in performing an adequate implant or increased risks of complications. Patients with locally extensive tumors—clinical stages T3 and T4—are generally not suitable candidates for isotope therapy except possibly as a boost technique. Brachytherapy is also starting to have a role in salvage treatment after localized recurrence for patients treated with definitive RT although the results from this form of treatment at this time are very limited (61,62,63,64,65).
Other Treatments
Cryotherapy, or freezing the prostate by circulating liquid nitrogen through interstitial catheters, is also utilized as curative treatment for clinically localized disease. Early results reported high complication rates although with newer techniques the complication rates have declined. Nevertheless, sexual dysfunction remains with near 100% incidence (35). A Canadian randomized trial did compare short-term ADT followed by either EBRT (68–73.5 Gy) or cryotherapy in men with localized prostate cancer where most (80%) had NCCN high-risk disease (66). Sexual dysfunction was significantly greater in those treated with cryotherapy (67). There were no significant differences in clinical outcome between treatment arms although a number of treatment features were suboptimal for the RT group including: low RT dose (most ≤70 Gy) (68,69), no pelvic RT (70), and only a short course of ADT (40,42). Each of these factors has been demonstrated alone to increase the rate of biochemical control for men treated with EBRT for prostate cancer. In addition, early repeat cryotherapy was not considered a treatment failure so a modest number of patients were retreated after biopsy-proven residual disease in the cryotherapy arm. Nevertheless, cryotherapy may be an option for patients with biopsy-proven recurrence after previous RT (71).
Noncurative options for prostate cancer include watchful waiting and active surveillance (both covered above) as well as androgen-deprivation hormonal therapy. Types of hormonal therapies include: orchiectomy, luteinizing hormone-releasing hormone (LHRH) agonists (e.g., leuprolide, goserelin) or antagonists (e.g., abarelix, degarelix), nonsteroidal anti-androgens (e.g., flutamide, bicalutamide) and estrogens. These hormonal agents can be used as debulking and anti-metastatic measures; however, these treatments are not curative unless combined with effective local therapy (43). They are, however, usually the first-line and most effective therapies available for disseminated disease. New systemic innovations are on the horizon with the recent reporting of the first cytotoxic chemotherapy agents that demonstrated improved survival in men with metastatic, hormone-refractory prostate cancer (72,73). In addition, newer hormonal agents are being developed which have resulted in promising PSA-based responses even in men with what was considered hormone refractory prostate cancer (74,75,76). The roles of these and other newer agents both alone and when combined with RT remain to be seen.
Treatment Planning for External Beam Radiation Therapy for Definitive Prostate Treatment
Target Volumes
Because prostate cancer is a multifocal disease and no current imaging modalities can reliably localize all areas of disease within the prostate, the prostate gland in its entirety is encompassed by the radiation prescription dose in current radiation oncology practice. The prostate is most commonly identified on a treatment planning CT; however, some have advocated for the use of MRI for treatment planning of the prostate (77). Although the prostate will always be treated, a clinical decision must be made as to whether the seminal vesicles and pelvic lymph nodes will be included. This decision depends on grossly demonstrated involvement of these structures or a substantial risk of being microscopically positive, as well as on institutional policy (see Table 26.3 for the criteria currently utilized at the University of Michigan).
The benefit of pelvic lymph node irradiation has been a controversial topic over many years (78); however, recent results from the Radiation Therapy Oncology Group clinical trial 94-13 provided insight (70,79). This trial included patients with an estimated likelihood of pelvic lymph node involvement of at least 15%. Patients were randomized to whole pelvic radiation versus prostate only radiation, as well as to short-term neoadjuvant and concurrent ADT versus adjuvant ADT starting at the completion of RT. No statistically significant differences were found in progression-free or overall survival between the two study questions that patients were randomized to: neoadjuvant ADT versus adjuvant ADT and pelvic RT compared with prostate only RT. Nevertheless, a trend toward improved progression-free survival was found in favor of the pelvic and neoadjuvant ADT arm compared with the other three arms. In addition, when the size of the pelvic field was separately evaluated, it appeared that there was a direct benefit when using a whole pelvic field as compared to a mini-pelvic field or prostate only treatment with 7-year progression-free survival of 40%, 35%, and 27%, respectively (p = 0.02) (80). Two other studies have come to negative conclusions in regard to the benefit of pelvic RT; however, these have been criticized as either having such a low risk of pelvic lymph node involvement that a benefit was unlikely to be seen (81) or to have been performed with ineffectual treatments in the pre-PSA era when local control was so poor as to render pelvic RT meaningless (82). Current imaging technology, including CT, MRI, and lymphangiograms, has limited accuracy for detecting pelvic lymph node metastasis. Therefore, until improved methods for detecting pelvic lymph node metastases are developed, clinicians will be limited to using risk factor assessment and clinical judgment in deciding whether pelvic lymph nodes should be encompassed in the target volumes.
Likewise, seminal vesicle involvement is associated with a relatively high risk of lymph node disease and a worse clinical outcome (83). The clinical risk factors of stage, GS, and PSA can be related to risk of seminal vesicle involvement, similar to lymph node risk (84). To date no study documents improved local control or survival if seminal vesicles are electively irradiated for the risk of clinically undetected microscopic disease; however, intuitively it seems important to include these structures in higher risk patients, so that likely potential sites of local disease in its entirety is treated. Some authors have presented evidence that only the proximal portion of the seminal vesicles adjacent to the prostate needs to be included in the target volume (85). Including the seminal vesicles, lymph nodes, or both within the target volumes increases the field size and may likewise increase the acute side effects and long-term complications (70).
Pelvic Lymph Nodes
For treating of pelvic lymph nodes traditional 2D treatment techniques commonly utilized four fields from the anterior, posterior, right, and left lateral orientations covering the targeted anatomical areas: the prostate, the seminal vesicles, and the pelvic nodes. Accurate targeting is critically important if 3D conformal or IMRT-based treatment techniques are utilized to treat the pelvic lymph nodes, yet there is significant heterogeneity in how pelvic lymph nodes are contoured even amongst experience genitourinary radiation oncologists (86). A recent consensus definition was established to make pelvic nodal volumes more uniform (87). Based upon this consensus, the pelvic lymph node volumes to be irradiated include: distal common
iliac, presacral lymph nodes (S(1)–S(3)), external iliac lymph nodes, internal iliac lymph nodes, and obturator lymph nodes. Lymph node CTVs include the vessels (artery and vein) and a 7-mm radial margin without extending into bowel, bladder, bone, and muscle. Volumes begin at the L5/S1 interspace and end at the superior aspect of the pubic bone (87). The treatment of presacral lymphatics is somewhat controversial as these nodes are infrequently problematic clinically, and are rarely an isolated site of recurrence if left untreated, and are not included in the standard pelvic lymph node dissection. Their inclusion in the radiation field necessitates that the posterior margin be expanded significantly, encompassing additional rectum and large bowel and unless using 3D conformal or IMRT techniques may result in increased irradiation of the rectum with increased risk of acute and long-term side effects. The role of RT in patients with known pelvic lymph node positive disease is less clear. However, as both RTOG 85-31 and the EORTC studies of high-risk prostate cancer allowed positive pelvic lymph nodes at diagnosis, treatment of patients with positive lymph nodes with both irradiation and ADT is a reasonable course of action.
iliac, presacral lymph nodes (S(1)–S(3)), external iliac lymph nodes, internal iliac lymph nodes, and obturator lymph nodes. Lymph node CTVs include the vessels (artery and vein) and a 7-mm radial margin without extending into bowel, bladder, bone, and muscle. Volumes begin at the L5/S1 interspace and end at the superior aspect of the pubic bone (87). The treatment of presacral lymphatics is somewhat controversial as these nodes are infrequently problematic clinically, and are rarely an isolated site of recurrence if left untreated, and are not included in the standard pelvic lymph node dissection. Their inclusion in the radiation field necessitates that the posterior margin be expanded significantly, encompassing additional rectum and large bowel and unless using 3D conformal or IMRT techniques may result in increased irradiation of the rectum with increased risk of acute and long-term side effects. The role of RT in patients with known pelvic lymph node positive disease is less clear. However, as both RTOG 85-31 and the EORTC studies of high-risk prostate cancer allowed positive pelvic lymph nodes at diagnosis, treatment of patients with positive lymph nodes with both irradiation and ADT is a reasonable course of action.
Prostate and Seminal Vesicles
The prostate is situated behind the pubic symphysis at midline in the anteroposterior (AP) view and behind the femoral head and proximal femoral shaft in the lateral projection. The seminal vesicles are superior and usually slightly posterior to the prostate gland, attaching at the base. With reference to the bony pelvis, these structures are typically ∼2 cm above the superior border of the pubis, although location can vary in any individual patient. The International Commission on Radiation Units and Measurements (ICRU) has defined volumes for treatment planning that take into account the extent of the known gross tumor, the areas of likely microscopic extension, and daily variations in patient setup and tumor position. These definitions of gross tumor volume (GTV), clinical target volume (CTV) and planning target volume (PTV) are given in Table 26.4 (88). Various institutions have adapted the ICRU definitions on tumor volumes for use in prostate cancer planning. In general, the prostate itself is considered the GTV. The GTV is then expanded by some amount to encompass potential microscopic extension of disease in the CTV. This may include all or some portion of the seminal vesicles, if they are at significant risk and are intended to be treated. Additional margin is added to the CTV to account for variation in daily treatment setup and organ motion to define the PTV.
The amount of margin added to the GTV to define the CTV and the PTV in this series of expansions has been the subject of extensive study and discussion. While it is important to make margins generous enough to consistently encompass gross and microscopic tumor by the prescribed dose, there is also compelling reason to keep the margins as small as possible near organs of limited tolerance, such as the rectum posteriorly, the bladder superiorly, and (possibly) the penile bulb inferiorly. In general, 0.5 to 1 cm is added around the prostate (and seminal vesicles) to ensure coverage of extracapsular microscopic tumor extension and to account for setup variability and organ motion. As an example, treatment guidelines for the recently closed RTOG clinical trial P0126 instruct that the GTV for intermediate-risk patients be defined as the prostate, the CTV be defined as the GTV plus the proximal 1 cm of the seminal vesicles, and the PTV be defined as the CTV plus a 0.5 to 1.0 cm margin depending on institutional policy. These volumes are then used for treatment planning using either 3D-CRT or IMRT techniques.
Table 26.4 International Commission on Radiation Units and Measurements Designated Tumor Volumes and Definitions | ||||||||
|---|---|---|---|---|---|---|---|---|
| ||||||||
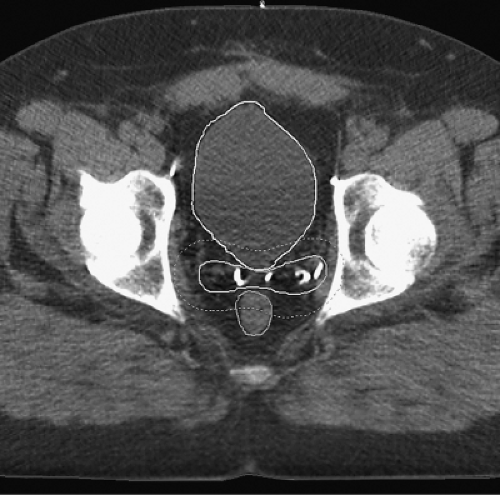
The PTV should be designed so that it covers the CTV with high probability for every treatment. Practitioners may adjust the size of the PTV depending on many factors that may influence the uncertainties in daily setup and prostate position including immobilization techniques, patient positioning, bladder and rectum filling, and others. Rigid immobilization devices such as the Alpha Cradle™ or thermoplastic body casts may increase setup accuracy. Rosenthal, et al. found that with immobilization only 1 of 10 patients had >0.5 cm variation in patient positioning for treatment, whereas 8 of 12 patients had more variation than this without immobilization (89). Improved positioning accuracy with rigid immobilization has been demonstrated in prospective randomized comparisons as well (90,91). Intra-rectal balloons may be expanded within the rectum to stabilize the prostate position, reducing the concerns over inter- and intra-fraction organ motion (92) (Fig. 26.1). However, use of these devices may be inconvenient for daily treatment and somewhat uncomfortable for the patient.
Instead of focusing on immobilization, many centers use daily imaging for prostate localization including daily CT (93), ultrasound (94), radiographic imaging of implanted radiopaque fiducial markers (95,96), or electro
magnetic transponders (97,98) (Fig. 26.2). Isocenter adjustments can then be made on a daily basis, ensuring adequate localization of the CTV at the start of treatment. These techniques fall under the category of a collection of adaptive RT practices termed IGRT (6). Using any of these IGRT techniques most authors have typically decreased prostate CTV to PTV margins to 0.5 to 0.75 cm with posterior margins of ∼0.5 cm.
magnetic transponders (97,98) (Fig. 26.2). Isocenter adjustments can then be made on a daily basis, ensuring adequate localization of the CTV at the start of treatment. These techniques fall under the category of a collection of adaptive RT practices termed IGRT (6). Using any of these IGRT techniques most authors have typically decreased prostate CTV to PTV margins to 0.5 to 0.75 cm with posterior margins of ∼0.5 cm.
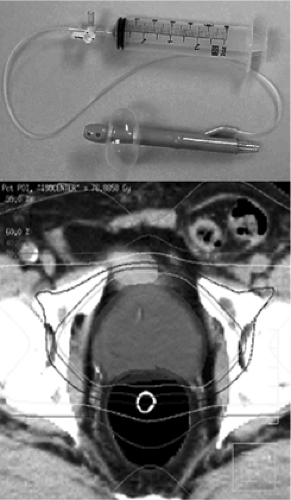 Figure 26.1. An intrarectal balloon device (top panel) can be used for stabilization of prostate position during external beam radiation therapy (bottom panel). Courtesy Dr. Mark Ritter. |
Prostate movement and the margin necessary to compensate for it have been studied in great detail. Ten Haken, et al. (99) reported maximal superior, inferior, anterior, and posterior prostate movement of 1, 1.25, 2, and 0.75 cm, respectively. The overall average movement was 0.5 cm, most often in the anterior and superior direction, while the average left-to-right movement was <0.05 cm. Nevertheless, residual errors in prostate position of over 7 mm after pretreatment localization and adjustment of prostate position clearly demonstrate the influence of intra-treatment movement. In a limited trial using fluoroscopy, Dawson et al. demonstrated breathing-related movement of the prostate, the magnitude of which increased with the amplitude of the breathing cycle (deep breathing vs. shallow) which even varied in the same patients based upon prone or supine positioning with greater movement in those evaluated prone (100). Just as observed by others the magnitude of movement was greatest in the anterior–posterior and cranial-caudal directions while minimal lateral movement was seen (101,102). Using non-IGRT techniques.
Even with IGRT for localization of the prostate at the start of treatment residual errors in position during and at the end of each treatment can occur due to intra-treatment motion of the prostate (99,100). Noel et al. recently evaluated posttreatment prostate position following initial localization at the start of treatment and found poor sensitivity to identify prostate motion unless it was evaluated more than two times a minute (103). Nevertheless, despite this poor ability to detect prostate motion the overall frequency of significant prostate motion may not be high. Li et al. recently evaluated motion in 105 prostate patients treated with electromagnetic localization of the prostate and found displacement of the prostate by more than 3 or 5 mm in 13.4% and 1.8% of treatment fractions for the whole population (104). Some patients, however, may be more susceptible to prostate motion for when they identified the 7 patients with the greatest motion during
treatment there would have been excursion of more than 3 and 5 mm in 41% and 15% of fractions, respectively (105). The use of electromagnetic transponders (Calypso™ beacons) allows for real-time evaluation of prostate during treatment to account for prostatic motion. Given the above studies, it is likely that this technology does not add significantly to other IGRT methods for the majority of patients. However, in a poorly identified minority it may provide a significant improvement in PTV treatment and sparing of organs at risk (OAR). Using electromagnetic transponders, we have at present used the same PTV margins (0.5 cm in all directions); however, with interruption of treatment if the prostate moves >0.3 cm from isocenter (105,106). Some have suggested that significantly smaller PTV margins may be applied with the use of Calypso beacons (105,107); while others have suggested that rotational effects take a greater role with smaller PTVs and have cautioned for a more conservative approach to PTV margin reduction even with real-time prostate motion evaluation (104). A prospective Phase 2 study did assess PTV margins of 3 mm and suggested that there was a smaller decline in patient reported quality of life at the end of treatment as compared to a historical control group (108), but it remains to be determined if this will translate into any long-term benefits. In addition, it is unclear if this smaller margin was delivered without a decrease in clinical efficacy and if the differences observed were due to the smaller margin size or other differences in either technique or patient characteristics.
treatment there would have been excursion of more than 3 and 5 mm in 41% and 15% of fractions, respectively (105). The use of electromagnetic transponders (Calypso™ beacons) allows for real-time evaluation of prostate during treatment to account for prostatic motion. Given the above studies, it is likely that this technology does not add significantly to other IGRT methods for the majority of patients. However, in a poorly identified minority it may provide a significant improvement in PTV treatment and sparing of organs at risk (OAR). Using electromagnetic transponders, we have at present used the same PTV margins (0.5 cm in all directions); however, with interruption of treatment if the prostate moves >0.3 cm from isocenter (105,106). Some have suggested that significantly smaller PTV margins may be applied with the use of Calypso beacons (105,107); while others have suggested that rotational effects take a greater role with smaller PTVs and have cautioned for a more conservative approach to PTV margin reduction even with real-time prostate motion evaluation (104). A prospective Phase 2 study did assess PTV margins of 3 mm and suggested that there was a smaller decline in patient reported quality of life at the end of treatment as compared to a historical control group (108), but it remains to be determined if this will translate into any long-term benefits. In addition, it is unclear if this smaller margin was delivered without a decrease in clinical efficacy and if the differences observed were due to the smaller margin size or other differences in either technique or patient characteristics.
Treatment Planning
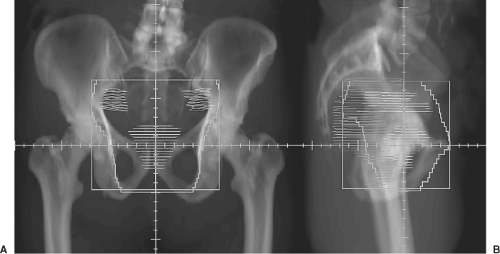
Once the process of defining the patient positioning and localization have been determined the process of treatment planning can commence. Most centers currently use forward planned, CT-based, 3D-CRT techniques, or inverse-planned IMRT. Although commonly utilized previously the added benefit of rectal, bladder, and/or urethral contrast when using CT-based planning is unclear. If the decision is made to treat the pelvic nodes, the classical superior bony landmarks in the AP view include the bottom of the L5 vertebral body to encompass the common, internal and external iliac nodes, midway through the sacroiliac joints to encompass the distal common, internal and external iliac lymph nodes or the bottom of the sacroiliac joints to encompass the internal and external iliac lymph nodes. The inferior margin is often set 1 cm below the ischial tuberosities inferiorly, and 1.5 to 2 cm to either side of the lateral bony pelvis laterally. In the lateral projection the anterior border is placed at the most anterior point of the pubis symphysis. The posterior border generally falls along the posterior ischium, although the field is usually shaped to bisect the rectum when filled with 20 to 30 mL barium GI contrast, administered via rectal tube. Retrograde urethrography may be performed using a small-gauge catheter to administer 5 to 10 mL of 30% Hypaque into the urethra with immediate penile clamping. The narrow point, or beak, in the contrast column denotes the level where the urethra passes through the urogenital diaphragm (109,110). The most inferior aspect of the prostate gland is typically located 1 cm above the urethral apex, and the inferior field margin is placed 1 cm below the apex or beak, allowing a 2-cm inferior margin on the prostate.
In most practices, CT planning has now largely supplanted the setting of fields using 2D fluoroscopic techniques. CT images facilitate the identification and localization of both target and normal tissue structures (87). Lymph node regions follow the path of the common iliac artery and its bifurcation into the internal and external iliac arteries, and can usually be easily traced even without intravenous contrast administration. Compared with the estimated general localization provided by bony landmarks in 2D planning, the size, shape, and location of the prostate and particularly the seminal vesicles can be visualized and defined more precisely with CT planning. The seminal vesicles drape around the rectum in some patients, and standard blocking in 2D fields set using rectal contrast may not provide adequate coverage of these structures. Identification of the prostate apex on axial CT is sometime difficult and may be facilitated with MRI or with sagittal and coronal reconstruction of CT data sets (111). Normal tissue structures including the rectum, bladder, loops of small bowel, penile bulb, and even important anatomic anomalies such as pelvic or horseshoe kidneys can be readily identified and localized with CT planning.
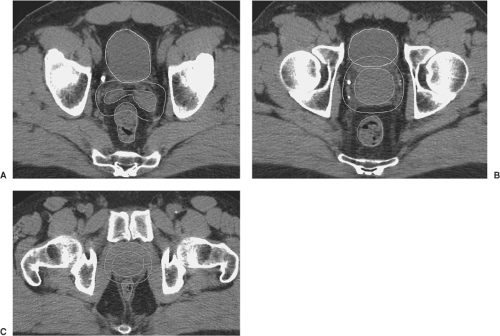
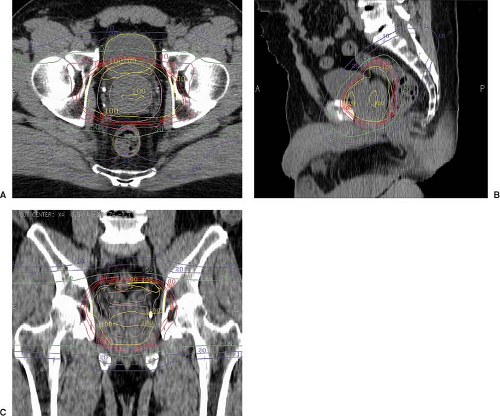
Fluoroscopic or CT simulation for treatment planning may be performed with the patient either supine or prone, with or without customized immobilization as discussed above. External marks are placed on the patient or immobilization device, often chosen to designate a preliminary isocenter position. These locations may be delineated with radiopaque markers taped to the patient in the x, y, and z axes that can be referenced to the image set, facilitating the transposition of the isocenter from the treatment plan to the patient at the time of treatment. For CT-based planning, CT images are obtained at 1 to 5 mm intervals through the levels to be encompassed within the treatment fields. Target and normal structures can then be defined and expanded to create the relevant PTVs on axial CT images within specialized treatment planning software (Fig. 26.3).
The issues regarding immobilization technique, patient positioning, and image guidance were discussed above. Common choices for immobilization include the Alpha Cradle™, thermoplastic cast material similar to Aquaplast, and “vacuum lock” molds. The CT scan must be done with the patient in the planned treatment position in the immobilization device, with attention toward patient comfort and maximization of daily reproducibility. Alternatively, for ease of position adjustment, some practitioners utilize supine position without an immobilization device if daily prostate localization techniques are employed (see above). An advantage of the prone position is to shift the seminal vesicles forward, away from the
rectum, potentially improving rectal sparing (112). On the other hand, prone positioning may be associated with more internal organ motion, including respiratory motion particularly in men with any degree of obesity (100). Roach et al. advocated nonuniform expansions around the prostate because of the variation in prostate movement depending on direction, the status of rectum and bladder filling as well as radiation tolerance of these two structures (113). The recommendations included anterior and inferior margins of 2 cm, lateral and superior margins of 1.5 cm, a posterior margin of 0.5 to 0.75 cm, and that CT planning be performed with the rectum empty and the bladder full. If bladder fullness is inconsistent from treatment to treatment, superior and inferior margins are more generous and accommodating, and this also helps to keep small bowel out of the field. If the rectum is empty when fields are planned, a rectum that is fuller during treatment only pushes the prostate further into the field, and 2 cm anterior margins should still provide adequate coverage of the CTV. However, with the use of image-guided therapy smaller margins may be appropriate (6).
rectum, potentially improving rectal sparing (112). On the other hand, prone positioning may be associated with more internal organ motion, including respiratory motion particularly in men with any degree of obesity (100). Roach et al. advocated nonuniform expansions around the prostate because of the variation in prostate movement depending on direction, the status of rectum and bladder filling as well as radiation tolerance of these two structures (113). The recommendations included anterior and inferior margins of 2 cm, lateral and superior margins of 1.5 cm, a posterior margin of 0.5 to 0.75 cm, and that CT planning be performed with the rectum empty and the bladder full. If bladder fullness is inconsistent from treatment to treatment, superior and inferior margins are more generous and accommodating, and this also helps to keep small bowel out of the field. If the rectum is empty when fields are planned, a rectum that is fuller during treatment only pushes the prostate further into the field, and 2 cm anterior margins should still provide adequate coverage of the CTV. However, with the use of image-guided therapy smaller margins may be appropriate (6).
Treatment Technique
Traditionally, a four-field technique is generally used to treat pelvic lymph nodes: anterior, posterior, right, and left lateral portals. Blocks can be drawn manually on simulation films or BEV digitally reconstructed radiographs (DRRs) from CT planning software. Construction of these blocked fields can be facilitated by reviewing the pelvic CT scan and entering lymph node volumes that track along the course of the iliac vasculature (Fig. 26.4). Depending on the external contour, which can be done manually or automatically by CT scan, small angle wedges on the lateral fields may help to produce a more symmetrical dose distribution. In addition, IMRT is being utilized with greater frequency to treat pelvic lymph nodes in which case prostate, seminal vesicles, pelvic lymph nodes, and OAR must all be defined for appropriate treatment planning and optimization. Examples of recommended OAR tolerances are provided (Table 26.5) (87).
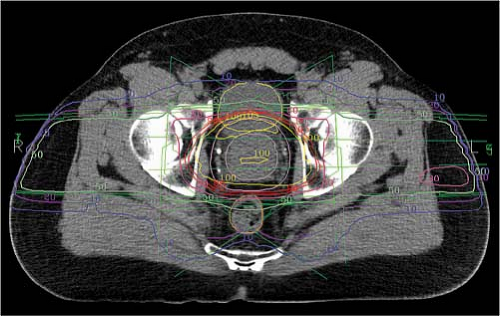
Two-dimensional planning to treat the prostate and seminal vesicles has classically used three fields (anterior, right, and left lateral fields), four fields (anterior, posterior, right, and left lateral fields), or even two bilateral 120° arc fields. With 2D planning, target volumes tend to be more generous and blocking more conservative to allow for the inaccuracies of manual transposition of PTVs to the simulation plain films. For CT-based planning, CT images are entered into a treatment planning software package. The lymph node regions, prostate, and seminal vesicles are outlined by the physician, and can be expanded by adding appropriate margins to establish the CTVs and PTVs. As noted previously, margins may vary between institutions and individual physicians and by the use of IGRT. Normal structures are also outlined including bladder, rectum, penile bulb, femoral heads, loops of small bowel or any other structures to which the physician may wish to limit dose. Treatment planning software packages allow manipulation of virtual 3D reconstructions of these volumes, allowing a variety of views for the planner, including “beam’s eye view” (BEV) perspectives. These features are designed to allow the user to manipulate beam orientation (gantry, table, and collimator angles) and shaping (blocking or multileaf collimation) to encompass targets and avoid normal structures. For forward-planned 3D conformal treatment planning, a combination of beams is devised complete with beam modifiers such as wedges or compensators. High-energy beams such as 10 to 18 MV are customary for pelvic lymph node and prostate irradiation, and the beams should certainly be 6 MV or greater. The software allows rapid calculation of the dose that can be displayed on the overlying images in the form of isodose lines or surfaces (Fig 26.5). Additional tools including dose-volume histograms (DVHs) helps the clinician to compare treatment plans to determine the optimal plan for coverage of target volumes and avoidance of normal tissue structures (Fig. 26.6).
Forward planned 3D CRT beam arrangements for prostate cancer treatment may include the standard axial anterior, posterior, and lateral beams common to 2D prostate plans, but can also easily make use of oblique and nonaxial beam angles. Rotation of the collimator is commonly used to place the orientation of the multi-leaf collimator appropriately so as to maximize normal tissue blocking and target coverage. Segmental fields, physical wedges and dynamic wedge techniques can be used to improve target coverage and dose homogeneity. In addition to the classic
four-field box technique, 3D CRT beam configurations include a six-field conformal technique (114) as well as a four-field variation using lateral and anterior obliques that eliminate the posterior-oblique beams. Marsh et al. have found that the four-field technique, using laterals and anterior–inferior obliques, often improves the rectal DVH compared with axial plans (115) (Fig 26.7). This technique requires supine patient positioning to achieve table clearance for the anterior–inferior beams.
four-field box technique, 3D CRT beam configurations include a six-field conformal technique (114) as well as a four-field variation using lateral and anterior obliques that eliminate the posterior-oblique beams. Marsh et al. have found that the four-field technique, using laterals and anterior–inferior obliques, often improves the rectal DVH compared with axial plans (115) (Fig 26.7). This technique requires supine patient positioning to achieve table clearance for the anterior–inferior beams.
Table 26.5 Dose Volume Histogram Constraints for Organs at Risk (87) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The application of IMRT technology to prostate cancer EBRT planning has enabled further improvements in normal tissue DVHs, mainly due to the ability of this technology to achieve convex dose distributions, reducing rectal dose (Fig. 26.8). The clinical significance of this advance toward reducing rectal toxicity remains undetermined although some groups have suggested that with the combination of IGRT and IMRT high dose EBRT can be delivered without increases in toxicity as compared to that observed in historical control groups treated to lower doses with less sophisticated techniques (5). For instance Zelefsky et al. noted an actuarial rate of grade 2 or greater rectal toxicity at 10 years of 13% in patients using 3D CRT and 5% using IMRT (p < 0.0001) (116) Of note IMRT patients received higher doses of RT than 3D conformal patients, but IGRT was also used with much greater frequency in the IMRT group as well. The inverse planning process starts with the contouring of target and normal tissue volumes on an axial CT image set—just as for 3D CRT planning. However, instead of inputting a candidate beam arrangement into the planning software and calculating a dose distribution for that plan, the IMRT software package uses an optimization algorithm to find a beam “solution” that maximizes a set of “cost functions.” Typically,
the process begins with a certain number of axial beam angles for the algorithm to utilize. Each beam is subdivided into square “beamlets” that represent the incremental movement of multileaf collimator leafs across the field in 1 cm or less increments. The algorithm “modulates” the fluence through each of these beamlets in an iterative process, evaluating and comparing each candidate plan to identify a better one. Each plan is evaluated for dose coverage of target volumes, as well as dose avoidance for normal tissue structures. Target coverage and normal tissue structure sparing is prioritized based on clinical judgment, and a cost for achieving or failing to achieve each objective is assigned. The algorithm will iterate until it identifies a plan that best achieves the stated objectives. Once the optimization algorithm produces a plan, a formal dose calculation is performed, and a quality assurance procedure is followed to ensure that the delivered dose will be within a desired level of accuracy. For the prostate, where the target shape and volume are relatively consistent from patient to patient, a template can be established to make the treatment planning process more efficient. Examples of typical dose constraints for rectum, bladder, femoral heads, and both large and small bowel are given in Table 26.5. The sparing of the penile bulb is controversial as different authors have come to differing conclusions about the utility of sparing the penile bulb in preserving sexual function (117). However, it is clear that if a penile bulb dose constraint is observed that it should not be at the cost of decreasing dose to the PTV. An example of an IMRT plan as well as dose volume histograms for both an IMRT plan and a 3D-CRT plan are given in Figure 26.9.
the process begins with a certain number of axial beam angles for the algorithm to utilize. Each beam is subdivided into square “beamlets” that represent the incremental movement of multileaf collimator leafs across the field in 1 cm or less increments. The algorithm “modulates” the fluence through each of these beamlets in an iterative process, evaluating and comparing each candidate plan to identify a better one. Each plan is evaluated for dose coverage of target volumes, as well as dose avoidance for normal tissue structures. Target coverage and normal tissue structure sparing is prioritized based on clinical judgment, and a cost for achieving or failing to achieve each objective is assigned. The algorithm will iterate until it identifies a plan that best achieves the stated objectives. Once the optimization algorithm produces a plan, a formal dose calculation is performed, and a quality assurance procedure is followed to ensure that the delivered dose will be within a desired level of accuracy. For the prostate, where the target shape and volume are relatively consistent from patient to patient, a template can be established to make the treatment planning process more efficient. Examples of typical dose constraints for rectum, bladder, femoral heads, and both large and small bowel are given in Table 26.5. The sparing of the penile bulb is controversial as different authors have come to differing conclusions about the utility of sparing the penile bulb in preserving sexual function (117). However, it is clear that if a penile bulb dose constraint is observed that it should not be at the cost of decreasing dose to the PTV. An example of an IMRT plan as well as dose volume histograms for both an IMRT plan and a 3D-CRT plan are given in Figure 26.9.
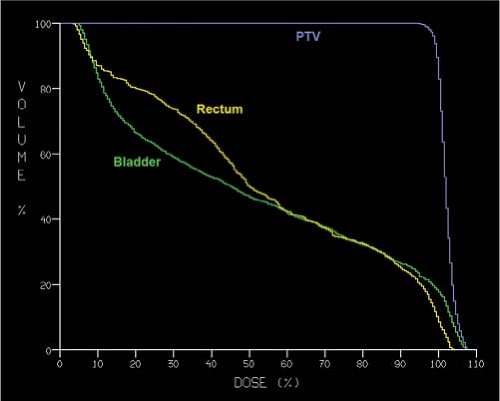 Figure 26.7. A typical dose-volume histogram (DVH) plot is shown for bladder, rectum and planning target volume (PTV) from a three-dimensional (3D) conformal radiation treatment plan. |
The sharp dose gradients that can be created using highly conformal techniques such as IMRT create a significant risk of missing the target due to inter- and intra-fraction setup uncertainty and organ motion. Therefore, highly conformal therapy should be combined with IGRT to ensure the accuracy of treatment and to minimize marginal misses due to setup error or changes in prostate position.
Dose and Fractionation
As advances in treatment techniques have allowed more conformal treatment of the target volumes with decreased dose to normal tissues, improved outcomes have been documented with increased doses to the prostate. However, the optimal treatment doses for various risk categories of prostate cancer remain unknown. With traditional 2D treatment techniques, doses of 45 to 50 Gy to the pelvis, 55 to 65 Gy to the seminal vesicles and 65 to 72 Gy to the prostate were typical. With the advent of 3D CRT, IMRT, and other highly conformal techniques, doses have been escalated without significant increases in acute side effects or late complications (118,119,120). In a landmark trial, the MD Anderson Cancer Center compared 70 to 78 Gy using 3D CRT techniques and found that patients receiving the higher dose had significant improved biochemical failure-free survival, particularly those patients with a pretreatment PSA above 10 ng/mL or with higher-risk clinical features (Table 26.6) (121). Toxicity; however,
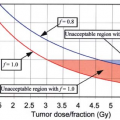
was increased as a function of the volume of rectum treated to higher doses. Another significant randomized trial of dose escalation used a combination of external beam photon radiotherapy with a proton beam boost. Zietman et al. have reported that for mostly low-intermediate risk prostate cancer patients receiving 79.2 Gy had a 15% absolute reduction in the risk of biochemical recurrence at 10-years as compared to those receiving 70.2 Gy (32.4% vs. 16.7%) (2). Given the lack of biologic difference between protons and photons there is no reason to presume that these results are unique to the use of a proton boost. Using this technique, although there was a slight increase in mild acute rectal toxicity, there was no significant difference in clinically substantial toxicity rates or patient reported quality of life (122). These randomized trials as well as others (123,124), combined with many retrospective studies, give strong evidence that dose escalation above 70 Gy may significantly improve prostate cancer biochemical cure rates. However, at present, dose escalated radiation has not resulted in clear differences in rates of metastasis, prostate cancer-specific death, or overall survival. Encouragingly, when dose-escalation is performed using conformal techniques toxicity rates seem to be acceptable (4,33,124). The potential interaction between dose-escalation and hormonal therapy in terms of disease control and toxicity has not yet been thoroughly explored although both the Dutch and British dose escalation studies included patients with short-term ADT; therefore, it is clear that dose escalation may still provide a benefit even in the setting of ADT use (123,124).
was increased as a function of the volume of rectum treated to higher doses. Another significant randomized trial of dose escalation used a combination of external beam photon radiotherapy with a proton beam boost. Zietman et al. have reported that for mostly low-intermediate risk prostate cancer patients receiving 79.2 Gy had a 15% absolute reduction in the risk of biochemical recurrence at 10-years as compared to those receiving 70.2 Gy (32.4% vs. 16.7%) (2). Given the lack of biologic difference between protons and photons there is no reason to presume that these results are unique to the use of a proton boost. Using this technique, although there was a slight increase in mild acute rectal toxicity, there was no significant difference in clinically substantial toxicity rates or patient reported quality of life (122). These randomized trials as well as others (123,124), combined with many retrospective studies, give strong evidence that dose escalation above 70 Gy may significantly improve prostate cancer biochemical cure rates. However, at present, dose escalated radiation has not resulted in clear differences in rates of metastasis, prostate cancer-specific death, or overall survival. Encouragingly, when dose-escalation is performed using conformal techniques toxicity rates seem to be acceptable (4,33,124). The potential interaction between dose-escalation and hormonal therapy in terms of disease control and toxicity has not yet been thoroughly explored although both the Dutch and British dose escalation studies included patients with short-term ADT; therefore, it is clear that dose escalation may still provide a benefit even in the setting of ADT use (123,124).
Table 26.6 Long-Term Follow-up of the MD Anderson Dose Escalation Trial: Biochemcial Freedom from Recurrence (Phoenix Definition) at 8 Years (121) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
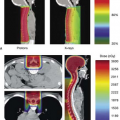
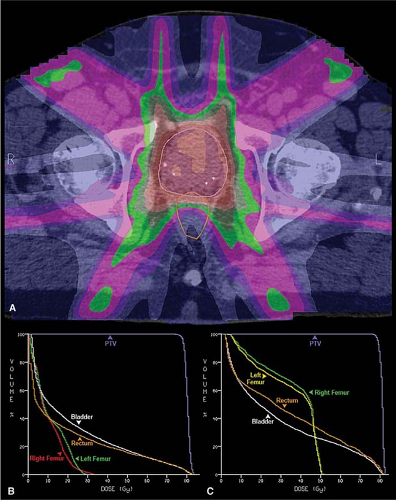 Figure 26.9. An axial computed tomography image is shown from a seven-field intensity modulated radiation therapy (IMRT) plan (A). The dose distribution is demonstrated using a dose “color-wash” and depicts the concave dose distribution achieved for the rectum. This results in the improved normal tissue dose volume histogram (DVH) for the IMRT plan (B) as compared with a four-field, three-dimensional (3D) conformal treatment plan DVH for the same patient (C).
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|