Cancers of the Gastrointestinal Tract
Matthew D. Callister
Jonathan B. Ashman
Introduction
Radiotherapy in combination with chemotherapy has a significant role in the treatment of cancers of the anus, rectum, pancreas, stomach, and esophagus. The role of combined-modality therapy in the treatment of these diseases has been defined largely as a result of several prospective clinical trials conducted over the past 20 years. This chapter reviews the technical aspects of treatment of gastrointestinal cancer, with particular emphasis on anatomic target definitions and modern treatment planning techniques.
Anal Cancer
In 2009, there were an estimated 5,290 new cases on anal cancer diagnosed in the United States (1). Given the favorable results of radiotherapy, either alone or in combination with chemotherapy, a sphincter-preserving approach is the standard of care for this disease.
Diagnostic Evaluation
The patient history and a thorough physical examination are essential for a successful treatment planning for patients with anal cancer. Risk factors for HIV infection should be reviewed with a low threshold for ordering laboratory testing if indicated. Careful review of gastrointestinal and genitourinary symptoms may indicate extension of tumor beyond what is clinically or radiographically detected.
The physical examination should give particular attention to the inguinal-femoral lymph nodes and anorectal canal. Inguinal nodes which contain gross metastatic disease on exam or imaging will be treated to a higher radiotherapeutic dose than areas requiring only elective treatment. Therefore, consideration should be given to fine needle aspiration of groin nodes of indeterminate size (≥1–1.5 cm) prior to treatment planning. Thorough examination of the perianal skin and anorectal canal is imperative for accurate treatment planning as it often reveals disease beyond what is visible on imaging studies alone. When patients have severe anal discomfort that inhibits adequate examination, such examination can be scheduled under anesthesia. Attention should be made to the proximal and distal extent of the tumor in relation to the anal margin as well as any perianal extension of disease. Among female patients, the radiation oncologist should perform a complete pelvic examination given the proximity of the anal canal to the lower posterior vaginal wall. In addition, the anatomic extent of disease reported on anoscopy or proctoscopy should be carefully reviewed.
Imaging studies should complement the history and physical examination in defining the extent of local-regional disease as well as detection of distant metastases. The abdomen and pelvis must be evaluated with either magnetic resonance imaging (MRI) or computed tomography (CT). Positron emission tomography (PET) may be of value in detecting regional or distant spread of disease not discernable by CT (2) and may alter radiotherapy field design (3). Endoscopic ultrasound (EUS) gives more accurate determination of the depth of tumor invasion than physical exam and may be of prognostic value (4), but is not required for treatment planning or staging.
Treatment Options
Patients with carcinoma of the anal canal should generally be treated with definitive radiotherapy. Chemotherapy is administered concurrently for all patients except those who are medically unfit or have very small tumors. The addition of mitomycin-C and 5-fluorouracil (5-FU) to radiotherapy has been shown to improve local control, colostomy-free survival, and disease-free survival (5,6,7). Patients who are HIV positive may be considered for combined-modality therapy if they are sufficiently immunocompetent (as defined by CD4 count). Carcinoma in situ and tumors of the perianal skin (which do not extend past the anal verge), should initially be considered for local excision. When these lesions cannot be excised adequately without significantly compromising anal function, definitive RT alone is an efficacious option. Abdominoperineal resection is reserved as salvage treatment for patients with persistent or recurrent disease after radiotherapy.
Treatment Planning
Simulation
The patient is simulated in the supine or prone position depending on the treatment technique selected (see section on “Radiotherapy Fields and Techniques”), though prone positioning may be less reproducible. The bladder
may be distended to decrease the amount of small bowel within the pelvis. To reduce skin toxicity, cutaneous folds within the medial groin may be minimized by moderately abducting the patient’s legs and using an immobilization device to ensure setup reproducibility of the lower extremities. The clinical extent of any visible or palpable perianal tumor should be demarcated with radiopaque markers as it may not be apparent on simulation imaging. The anal verge should also be marked and the rectum may be visualized with rectal contrast. The amount of exposed anal tumor posteriorly can vary significantly depending on degree of perineal extension, body habitus, and leg positioning. This should be noted at the time of simulation to ensure proper beam selection and occasionally bolus may be indicated with posterior beams in a thin patient due to exposed tumor. Oral contrast assists in small-bowel visualization. The use of CT-based targeting and treatment planning is critical for accurate treatment of the inguinal lymph nodes and to complement the findings of physical examination in regards to the extent of the primary tumor.
may be distended to decrease the amount of small bowel within the pelvis. To reduce skin toxicity, cutaneous folds within the medial groin may be minimized by moderately abducting the patient’s legs and using an immobilization device to ensure setup reproducibility of the lower extremities. The clinical extent of any visible or palpable perianal tumor should be demarcated with radiopaque markers as it may not be apparent on simulation imaging. The anal verge should also be marked and the rectum may be visualized with rectal contrast. The amount of exposed anal tumor posteriorly can vary significantly depending on degree of perineal extension, body habitus, and leg positioning. This should be noted at the time of simulation to ensure proper beam selection and occasionally bolus may be indicated with posterior beams in a thin patient due to exposed tumor. Oral contrast assists in small-bowel visualization. The use of CT-based targeting and treatment planning is critical for accurate treatment of the inguinal lymph nodes and to complement the findings of physical examination in regards to the extent of the primary tumor.
Radiotherapy Target
The radiotherapy target includes the primary tumor, clinically involved lymph nodes, as well as perianal tissues and regional lymphatics at risk for subclinical spread of disease. All diagnostic information should be used together (imaging, physical exam, and endoscopy) to define the maximal extent of the gross tumor volume (GTV) for the primary lesion and any involved lymph nodes. Treatment planning that relies solely on the images from a planning CT risks underestimation of the full extent of tumor involvement.
The clinical target volume(s) includes inguinal-pelvic lymphatics and perianal tissues. Adjacent to the primary anal tumor, the CTV should include a 1 to 2 cm radial margin of surrounding soft tissue. Longitudinally, it should include any portions of the anal canal not involved by tumor, including at least 2 cm of normal mucosal or skin margin. The CTV also includes the inguinal and pelvic lymph nodes. Data from abdominoperineal resection series document a pelvic nodal involvement rate of 35% and a subsequent inguinal nodal failure rate of 13% (8) (without dissection). Similarly, an inguinal nodal failure rate of 15% is reported in a radiotherapy series without elective groin treatment (9).
Traditional radiotherapy fields have included inguinal lymph nodes generally based on bony landmarks and palpation. Accurate definition of this nodal station by CT imaging requires more than just identifying the femoral blood vessels. The inguinal lymph nodes are located within the fatty tissue throughout the femoral triangle. The medial border of this triangle is the pubic tubercle and the adductor longus muscle. The lateral border is the sartorius muscle, including the fatty tissues underneath this muscle. The inferior apex of the femoral triangle is where the sartorius and adductor longus muscle intersect in the sagittal plane (generally where the deep branches of the femoral artery and vein originate). The superior extent of the femoral triangle is the inguinal ligament. The ligament is difficult to delineate by CT, but can be reproduced as a line from the pubic tubercle to the anterior superior iliac spine. The floor of the femoral triangle is the flexor muscles of the hip, but the lymph nodes lay in the fatty tissue at and anterior to the level of the femoral vessels. Extension of radiotherapy fields to cover the far lateral inguinal region adjacent to the anterior superior iliac spine may be of little benefit based on lymphangiogram and surgical dissection data (10).
Pelvic lymph nodes included in the CTV are the external and internal iliac, perirectal, and presacral nodal regions. As the superior extent of traditional fields for pelvic nodal treatment is generally the L5–S1 interspace, the lower portion of the common iliac lymph nodes may be included as well. In defining these nodal areas, contouring should not be limited to pelvic vasculature alone. The adjacent soft tissues of the pelvic sidewall and presacral area must be included (see section on “Radiotherapy Target” under section “Rectal Cancer”). A Radiation Therapy Oncology Group (RTOG) panel prepared detailed excellent review of the CTV for lower gastrointestinal cancers which serves as an excellent reference in target definition during treatment planning (11).
The planning target volume (PTV) should be an expansion of the CTV sufficient to account for organ motion and patient setup error. A minimum PTV of 1 cm beyond the CTV is appropriate, though smaller margins may be used if image-guided radiotherapy (IGRT) is employed.
Normal Tissue Tolerances
The amount of small bowel treated to >45 Gy should be minimized. Although this is generally achievable due to the inferior location of the boost target volume, large volumes of bowel receiving even moderate doses of radiation (as low as 15–30 Gy) probably contribute to acute toxicity (12) and should be minimized when possible. The femoral heads and necks should receive <45 Gy (13), and preferably <40 Gy. The perianal skin develops significant moist desquamation as a result of combined-modality therapy. Dosimetric hot spots in this region should be avoided. Although treatment breaks are commonly employed in the treatment of anal cancer due to acute skin toxicity (as well as gastrointestinal and hematological side effects), prolongation of overall treatment time has been associated with a detriment in local control (14,15). In the absence of infection, treatment breaks for skin toxicity alone should not be systematically scheduled, but instead should be avoided when possible, with aggressive supportive measures.
Radiotherapy Dose
The appropriate dose for elective nodal sites has not been well defined. In RTOG 87-04, 30.6 Gy in 1.8 Gy fractions was delivered to initial pelvic fields with the superior border placed at the L4–L5 interspace (5). Subsequently, the superior field borders were reduced to the bottom of the sacroiliac joints, and the pelvis and inguinal nodes continued treatment to 36 Gy. Finally, 10 × 10 cm fields which included the primary tumor and lowermost pelvis were
treated to cumulative doses of 45 to 50.4 Gy. In RTOG 98–11, the initial pelvic fields were treated to 30.6 Gy with the superior border placed at L5–S1 (16). Reducing the superior border to the bottom of the sacroiliac joints, the lower pelvis was treated to 45 Gy. The inguinal lymph nodes electively received 36 Gy. Although successive pelvic field reductions probably address the gradient of risk for micrometastatic disease within lymph nodes, retrospective series suggest that doses as low as 30 Gy with concurrent chemotherapy may be sufficient to achieve control of subclinical disease within nodes or at the primary site after excisional biopsy of small lesions (17,18,19). When concurrently treating multiple targets at different doses per fraction (i.e., IMRT), higher total doses may be indicated for regions receiving <1.8 Gy/day (20).
treated to cumulative doses of 45 to 50.4 Gy. In RTOG 98–11, the initial pelvic fields were treated to 30.6 Gy with the superior border placed at L5–S1 (16). Reducing the superior border to the bottom of the sacroiliac joints, the lower pelvis was treated to 45 Gy. The inguinal lymph nodes electively received 36 Gy. Although successive pelvic field reductions probably address the gradient of risk for micrometastatic disease within lymph nodes, retrospective series suggest that doses as low as 30 Gy with concurrent chemotherapy may be sufficient to achieve control of subclinical disease within nodes or at the primary site after excisional biopsy of small lesions (17,18,19). When concurrently treating multiple targets at different doses per fraction (i.e., IMRT), higher total doses may be indicated for regions receiving <1.8 Gy/day (20).
Only small primary tumors that have completely responded to radiation should be treated to 45 to 50.4 Gy. Larger tumors (T3/T4) and incompletely responding tumors should receive 54 to 59 Gy. Multiple retrospective series have reported superior local control rates in this dose range compared to lower doses (9,17,21). For patients treated with radiotherapy alone, the primary tumor should receive a cumulative dose of 60 to 63 Gy (22).
Radiotherapy Fields and Techniques
The treatment of patients with anal cancer can be technically challenging given the complex geometric distribution of targets, particularly the inguinal nodes, in relationship to normal pelvic structures. Most of the reported techniques have been devised to enable safe treatment of the inguinal lymph nodes overlying the femoral heads, while there has been less emphasis on techniques to minimize unnecessary dose to the genitalia, bladder, and small bowel. The risk of femoral neck fracture is rare at doses below 45 Gy, but significantly increases after 50 Gy (13). Treatment designed to treat inguinal lymph nodes but spare the femoral head and neck should be done cautiously. The deep inguinal lymph nodes (defined at the depth of the femoral vessels) are generally much deeper than has been historically appreciated. These nodes are routinely deeper than 3 cm and in the majority of patients may lie 5 to 6 cm from the surface (23,24,25), beyond the effective treatment depth of some electron techniques. Care must be taken to determine the depth of the inguinal vessels on the treatment planning CT and choose an appropriate treatment technique. Patient body habitus may influence not only depth of lymph nodes, but also the selection of the optimal technique for delivery of radiation (26).
Below is discussion of some of the common external beam techniques for treating anal cancer. Each approach has relative advantages and disadvantages, which are somewhat dependent on the radiation dose prescribed to elective nodes.
3D/IMRT: There are only limited reports of treatment of anal cancer with more sophisticated treatment planning techniques, but improvements in conformality of dose to targets could lead to reductions in normal tissue complications. Vuong et al. (27) have reported encouraging acute toxicity outcome among 30 patients with anal cancer treated with a complex 3D conformal technique.
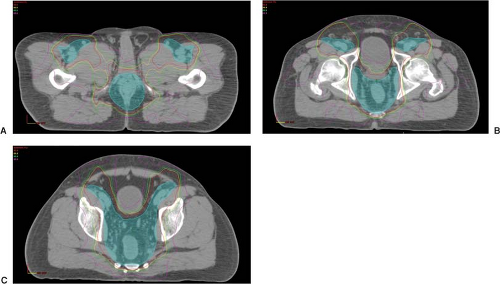
Initial dosimetric analysis of the use of IMRT in treating anal cancer has also shown promise in regards to conformality and sparing of the genitalia, small bowel, bladder, bone marrow, and femoral necks (see Fig. 24.1)
(28,29,30,31). Actual treatment with IMRT has been reported in a limited number of series with favorable toxicity and clinical outcomes (32,33). Preliminary results of RTOG 0529, a phase II trial of IMRT with concurrent chemotherapy, showed an encouraging reduction in the expected rate of gastrointestinal, genitourinary, and dermatologic toxicity (20). While attempting to spare normal tissues with these techniques, precise contouring of treatment targets is critical in order to avoid inadvertently undertreating regions of risk previously included in clinical trials.
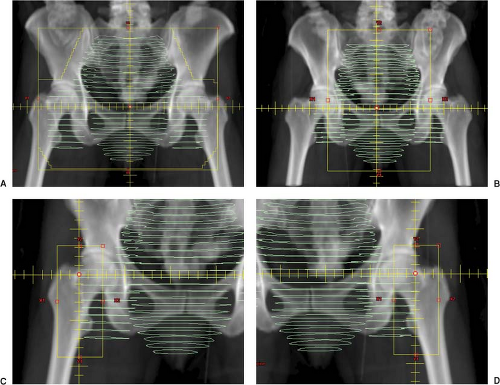
Wide AP/Narrow PA (see Fig. 24.2): A wide AP photon field, which includes the inguinal lymph nodes is simulated with a narrow PA field that excludes the femoral neck (may include a small sliver of the medial femoral head for margin on the obturator nodes). Due to divergence, it is occasionally possible to match the lateral exit of the narrow PA beam with the surface entrance of the wide AP photon beam and provide sufficient dosimetric coverage of the groins (26). More commonly, however, supplemental anterior electrons are indicated. The lateral exit of the narrow PA field is marked anteriorly on the patient’s groins (easily done at time of fluoroscopic simulation). This serves as the medial border for each electron supplement. The lateral border of each electron supplement is placed at or 1 cm lateral (due to beam constriction) to the surface entrance of the wide anterior photon beam. Particular attention must be made during treatment to any shifts made with the PA field to ensure similar movement in the anterior electron field junctions. Radiopaque wire place on these junctions may assist proper setup when porting the PA field. To reduce the complexities associated with electron groin setups, photons have also been used to supplement the lateral inguinal lymph nodes using the same AP photon field and setup. This can be achieved by the use of a central (pelvic) partial-transmission block (34) or with multi-leaf collimation (35,36).
Wide AP/Wide PA: Photon fields are devised to encompass the primary tumor and pelvis and laterally extend to encompass the inguinal lymph nodes (“wide”) in both the AP and PA fields (13,17). This field arrangement is the most simple as it requires no field matching, reducing the chance of technical error or underdosing the inguinal lymph nodes. Without the use of electrons, patients can be treated in the prone position, possibly reducing the amount of small bowel in the pelvis. The obvious disadvantage is that there is no sparing of the femoral neck, genitalia, and bladder. However, if moderate doses are selected for elective nodal irradiation, doses to these structures may be kept well within organ tolerance. Series from the M.D. Anderson Cancer Center have reported the use of this technique (17,37). The initial 30.6 Gy of radiation (pelvis and groins) is delivered with wide photon AP–PA fields. Subsequently the patient’s lower pelvis is resimulated with prone positioning on a belly-board using a three-field technique (narrow PA excluding lateral groins and lateral fields) for an additional 19.8 Gy. During this phase, inguinal lymph nodes are supplemented with anterior electrons (patient flipped supine), only if initially involved with tumor. Finally, the primary tumor is boosted to an appropriate final dose.
Four-field Technique: With this technique, three of the photon fields (AP and laterals) include the pelvis and inguinal region while the PA field is kept “narrow” to exclude the femurs. With appropriate beam weighting and energy selection, sufficient dose can usually be delivered to the inguinal nodes, as verified with computerized treatment planning. Patients may be treated in the prone position, reducing bowel within the pelvis. There are two variations of a four-field technique. First, an AP field is simulated “wide” and the PA field is kept “narrow” as above. The lateral fields, however, exclude the inguinal region (anterior border is placed at the pubic symphysis) and anterior electrons groin fields are then used to supplement the wide AP field (38). In the other variation, the patient may be treated prone on a belly-board with a wide PA field which exists through the groins and lateral fields which exclude the inguinal lymph nodes (three fields) (39). Supplementation of the groins with anterior electron fields is administered in the supine position. Although these techniques often reduce dose to the medial inguinal skin and genitalia, they both require complex setup and creation of a somewhat imprecise internal junction between the distal electron dose gradient and the lateral photon beams edges.
Boost Planning
With the exception of dose-painted intensity-modulated radiation therapy (IMRT), the GTV (both the primary tumor and the involved lymph nodes) should be boosted with reduced fields encompassing tumor with a 2 to 3 cm margin to the field edge. Using 3D definitions, a boost PTV of at least 1 to 1.5 cm around the GTV is appropriate with additional dosimetric margin to the field edge. When possible, a composite representation of dose between the initial and boost treatments should be created to ensure normal organ tolerance is not exceeded during the boost phase of treatment. A variety of beam arrangements may be used. Separate boost fields for involved inguinal lymph nodes are usually necessary but care should be taken not to concurrently overlap any tissues irradiated by the boost treatment to the anus. Boosting the primary lesion with a perineal field precludes CT planning in the treatment position and should be reserved for tumors that can be completely exposed to ensure coverage (e.g., tumors of the anal margin).
Chemotherapy
Careful coordination of the timing of concurrent chemotherapy with the delivery of radiation is important for successful treatment of anal cancer. The addition of mitomycin-C (10 mg/m2 on days 1 and 29 of radiotherapy) to 5-FU (1 g/m2/day for 96 hours on days 1–4 and 29–32 of radiotherapy) was established as the standard of care by the results of RTOG 87-04 (5), which compared both drugs to concurrent 5-FU alone. Treatment should begin on a Monday or Tuesday so that radiotherapy may be given on each day of chemotherapy delivery (4 days of 5-FU). As an alternative to mitomycin-C, multiple institutions have reported excellent results and with cisplatin and 5-FU concurrent with radiation (37,40). However, in RTOG 98-11, cisplatin and 5-FU, given as induction therapy and concurrent with RT, have not been found superior to RT concurrent with mitomycin-C and 5-FU (16).
Prognosis
The outcome of patients treated with combined-modality therapy is well described from the results of randomized trials (5,6,7,16). Favorable outcome is somewhat dependent clinical stage but long-term local control, colostomy-free survival, and overall survival are approximately 70%, 70%, and 60% to 70%, respectively among patients receiving combined-modality therapy. Among patients who fail treatment locally, 50% have been salvaged with surgical resection (5).
Colorectal Cancer
In 2009, there were an estimated 106,100 cancers of the colon and 40,870 cancers of the rectum diagnosed in the United States (1). Collectively, these cancers account for 9% of overall cancer incidence and death among both men and women. Radiotherapy plays an important neoadjuvant or adjuvant role in the management of rectal cancers, which penetrate bowel wall or spread to adjacent lymph nodes, but is uncommonly indicated in the treatment of colon cancer.
Diagnostic Evaluation
Careful diagnostic evaluation is essential not only for accurate staging and selection of patients for neoadjuvant strategies, but also to assist appropriate target definition during treatment planning. During the patient history, careful attention should be given not only to bowel-related symptoms, but also to indicators of local tumor extension into other pelvic structures (bladder/prostate or pelvic wall). The radiation oncologist’s digital rectal examination should assess the distance of the tumor from the anal verge, size and location, degree of circumferential involvement, and palpable morphology. The mobility of palpable lesions is described as mobile, tethered, or fixed, the latter implying initial unresectability. Proximal rectal cancers that are not palpable are referenced to the anal verge by endoscopy. Measurements from flexible endoscopies should be relied upon with caution as they are notoriously imprecise and inconsistent between exams. Measurements from rigid proctoscopy tend to be more reliable.
Pretreatment imaging is not only important to rule out distant metastases, but also to determine patients who are candidates for neoadjuvant chemoradiotherapy. EUS or MRI has been reported superior to CT in both determination of tumor depth of penetration (T stage) and detection of perirectal adenopathy (41). High-resolution MRI may be of particular benefit in predicting involvement of circumferential resection margin (42,43). Pelvic CT or MRI is indicated for the evaluation of pelvic lymphatic stations beyond perirectal lymph nodes. Among patients with locally advanced disease, PET has detected liver metastases in up to 8% of patients, which were not identified by other modalities (44). PET does not have sufficient spatial resolution to determine depth of tumor penetration nor consistently distinguish perirectal adenopathy from adjacent tumor.
Treatment Options
The use of adjuvant or neoadjuvant radiotherapy is generally reserved for patients whose tumors penetrate the bowel wall (≥T3) or are node positive. Among such patients, randomized trials clearly support the benefit adjuvant combined-modality therapy (pelvic radiotherapy and 5-FU-based chemotherapy) in regards to both local control and overall survival (45,46,47). The German Rectal Cancer Study Group reported improvement in local control with preoperative chemoradiotherapy versus similar postoperative treatment (5-year local relapse rate of 6% vs. 13%, p = 0.006) (48). In addition, randomized trials of hypofractionated pelvic radiotherapy versus surgery alone have shown improvement in overall survival (49) as well as local control, even among patients undergoing total mesorectal excision (TME) (50). The presence of node positivity or penetration of tumor through the bowel wall needs to be determined by EUS or MRI in order to justify a neoadjuvant approach.
Adjuvant radiotherapy for patients with colon cancer is not indicated. Although retrospective series have shown favorable local control outcomes with the use of adjuvant radiotherapy among patients with T4 tumors and selected T3 tumors, and positive lymph nodes (51,52), this has not be corroborated in clinical trials. Among such patients, Martenson et al. (53) reported the results of a randomized trial, which studied the use of adjuvant chemoradiotherapy but did not detect a benefit to patient survival. Preoperative treatment may be considered in patients whose tumors are considered unresectable at presentation.
Treatment Planning
Simulation
Proper patient positioning can significantly reduce the amount of small bowel within the pelvis and limit toxicity from treatment. In addition to decreasing the volume of bowel receiving maximal organ tolerance (45–50 Gy), reduction of bowel receiving doses as low as 5 Gy has been associated with improved patient tolerance to pelvic radiation (12,54,55). Maximal displacement of small bowel out of the pelvis is achieved with prone positioning and bladder distension. Without the use of a belly-board or lower abdominal wall compression, however, ∼25% of patients will have more small bowel in the pelvis with prone positioning compared to supine (more commonly in obese patients) (56). Multiple studies have documented significant reduction in pelvic small-bowel volume with use of a belly-board device (57,58,59) or lower abdominal wall compression (56). Bladder distension may actually contribute more to bowel displacement than a belly-board, but the effect of each method appears additive (60) and concurrent use of both techniques should be considered. Although these efforts displace bowel from the pelvis, they may also be less reproducible and be associated with variation in patient setup, which is undesirable when employing highly conformal radiation techniques.
Although small-bowel series are useful during fluoroscopic simulation, CT-based planning provides more complete identification of bowel, especially any that does not opacify with contrast (61). Small bowel has been shown to be more mobile in patients treated preoperatively than after surgical intervention (61). To ensure there has been no significant change in bowel positioning during treatment, consideration may be given to repeat CT-imaging at the time of boost planning for patients whose total pelvic radiation doses approach or exceed bowel tolerance.
Simulation in the prone position enables access for digital rectal examination. This allows verification of lower and middle rectal lesion location in relationship to the anal verge, while maintaining the treatment position. The anal verge should be marked with a radiopaque marker and rectal contrast instilled into the rectum to assist radiographic identification of the primary lesion. Insufflation of a small amount of air into the rectum (50 cc) may further assist in identification of the rectal mass (62). Taping the buttocks laterally may reduce their self-bolusing effect on the perianal skin during treatment, but is of
questionable reproducibility. Patients who have undergone abdominoperineal resection must have the perineal scar marked and included in the initial pelvic fields, with appropriate bolus utilized posteriorly if exposed. Perineal recurrence has been described in 8% to 30% of patients in the absence of adjuvant RT (63,64,65), but as low as 2% when the scar is adequately treated (66).
questionable reproducibility. Patients who have undergone abdominoperineal resection must have the perineal scar marked and included in the initial pelvic fields, with appropriate bolus utilized posteriorly if exposed. Perineal recurrence has been described in 8% to 30% of patients in the absence of adjuvant RT (63,64,65), but as low as 2% when the scar is adequately treated (66).
Radiotherapy Target
For patients treated preoperatively, the GTV includes the primary tumor and any radiographically enlarged lymph nodes. All clinical, endoscopic, and imaging information must be used to define the maximal extent of the rectal tumor. Relying solely on one modality risks underestimation of the tumor extent and inaccurate field design (especially within boost portals). Although not mandatory in treatment planning, the addition of PET to CT in GTV contouring has been shown to increase the target size by an average of 25% (67) and lead to change in treatment fields in 17% of patients (68). Soft-tissue extension or suspected infiltration of rectal tumors into adjacent mesorectal fat should be included within the GTV, particularly posteriorly.
The CTV encompasses all the perirectal tissue, presacral space, and lymphatics of the internal iliac chain (which are not commonly dissected at the time of surgery). A report of 269 cases of rectal cancer that recurred in the pelvis after surgery alone emphasized the predilection for rectal cancer recurrence in the posterior pelvis, specifically the presacral space. Among patients undergoing anterior resection, 93% recurred at or posterior to the colorectal anastomosis (69). Other studies of rectal cancer failure patterns have also shown infrequent recurrence above the S1–S2 interspace, lateral pelvic nodes, or in the anal sphincter (70,71).
Accurate contouring of the pelvic CTV involves more than simply identifying the internal iliac vessels. A study comparing locations of external and common pelvic lymphatics (by lymphangiography) to vessel location revealed that lymph nodes are not directly superimposable upon vasculature (72). Myerson et al. (62) and Roels et al. (73) provide an excellent review of CT-based treatment planning for rectal cancer. In the upper pelvis, the CTV should extend cephalad to include the sacral promontory, posteriorly to include the anterior wall of the sacrum, and laterally it should encompass vasculature and presacral soft tissue to the border of the iliopsoas muscles. In the mid-pelvis, the CTV includes similar tissues with care taken to include perirectal fat anterior to the rectum. In addition, 1 to 2 cm of posterior bladder or uterus may be included if at risk of subclinical extension of disease for patients who have adjacent, locally advanced lesions. In the lower pelvis, the CTV includes all the perirectal fat inferiorly and laterally extending to the levator ani muscles. It should extend to the posterior wall of the prostate or vagina as well. A larger margin of anterior pelvic organs is indicated for tumors with documented invasion (T4). The CTV should also include 2 cm of normal rectum cephalad and caudad to the primary tumor. With a subsequent PTV expansion of at least 1 cm (without IGRT) around the CTV, this will provide a minimum 4 cm longitudinal margin from GTV to block edge (assuming an additional 1 cm beyond PTV to block edge for dosimetric buildup). Given the distensibility of the rectum, more PTV margin may be indicated anteriorly, especially for anterior wall tumors of the mid- and upper rectum. Bladder, small bowel, and femoral heads should also be contoured for evaluation of normal tissue tolerance.
The inclusion of the external iliac lymph nodes is generally reserved for patients with T4 tumors, which extend into anterior organs of the pelvis (prostate, bladder, vagina, and cervix/uterus) for whom these nodes are at risk. This modification is supported by failure patterns (69) from patients treated with surgery alone. However, in a series of patients with T4 rectal cancers where external iliac lymph nodes were not routinely included within RT portals, regional recurrence of disease still occurred almost exclusively within the radiotherapy field (74).
Tumors of the lower rectum, which extend to the dentate line of the anal canal, have a theoretical risk of failure in the inguinal lymph nodes. A report of 184 patients with such lesions revealed only six groin failures (5-year actuarial rate of 4%) without elective radiotherapy to the inguinal lymph nodes (75). Thus, for patients with low-lying rectal cancers, careful attention should be given to the groins during initial staging, but the added toxicity of treatment does not seem justified if the inguinal nodes are clinically negative.
In the postoperative setting, treatment volumes are similar except that with the removal of the primary lesion, the entire preoperative tumor bed must be reconstructed and included within the CTV (often identified separately as a boost volume—CTV2). Review of preoperative imaging, operative reports and surgical clip placement is imperative. As mentioned above, the perineal scar must be included in the initial fields for patients who have undergone abdominoperineal resection.
To account for internal target motion and patient setup variability, a PTV is delineated beyond the CTV. In the absence of IGRT, PTV margin of at least 1 cm is appropriate. However, retrospective study of interfraction variability has shown that in the patient setup without IGRT can easily approach these margins, especially among patients with large body mass index treated in the prone position (76). Intrafraction movement of the mesorectum has also been evaluated and found on average to move <4 mm in various dimensions, but significant individual variability is observed (77).
Normal Tissue Tolerances
The amount of small bowel receiving 45 Gy should be minimized without compromising target coverage. Any small bowel within the boost volume of treatment should be limited and not receive beyond 50 Gy. In addition to maximum dose, acute intestinal side effects from pelvic radiotherapy have been found to correlate with volume of bowel receiving all dose levels to as low as 5 Gy (54,55). Thus, bowel exposure at any dose should be minimized.
The femoral heads and necks should receive <45 Gy (13), and preferably <40 Gy. Blocking in the lateral fields excludes the anterior genitalia in addition to small bowel.
The femoral heads and necks should receive <45 Gy (13), and preferably <40 Gy. Blocking in the lateral fields excludes the anterior genitalia in addition to small bowel.
Radiotherapy Dose
There is very little data in literature regarding the optimal radiotherapy dose in the treatment of rectal cancer. One retrospective study supporting current dosing guidelines is from Brizel and Timmerman (78) who observed superior local control with adjuvant radiotherapy doses ≥45 Gy compared to patients who received less dose. Forty-five Gy, given in 1.8 Gy fractions, is the currently accepted dose and fractionation for initial pelvic fields based on its repeated use in clinic trial designs (47,79). After the initial 45 Gy, a boost of 5.4 Gy is generally given to the GTV in preoperative cases and a 5.4 to 9 Gy boost is delivered to the tumor bed (CTV2) in the postoperative setting with careful attention to minimize small bowel within the field. Treating beyond a cumulative dose of 50 Gy should only be considered when small bowel can be completely excluded from this high-dose region of treatment.
Radiotherapy Fields and Techniques
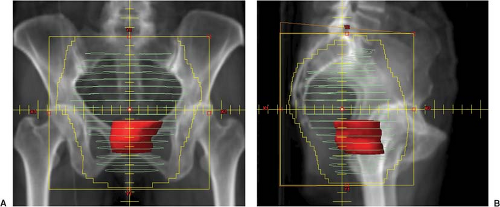
Patients are commonly treated with a three-field technique consisting of a PA and laterals (see Fig. 24.3). When inclusion of the anterior pelvis is indicated (e.g., treatment of the external iliac lymph nodes), an additional AP field may be advantageous dosimetrically. High energy photons and appropriate beam wedging and weighting are mandatory to ensure a homogenous dose distribution within the pelvis.
Traditional field design has been based on boney landmarks, the location of contrast-enhanced bowel/rectum and the anal verge. The superior border of the PA (and AP) fields generally covers the sacral promontory while the inferior border is placed at least 3 to 4 cm distal to the rectal cancer. For upper rectal cancers, the distal border need not include the entire anal canal, but should extend to the dentate line (about 2 cm from the anal verge) so that all the mesorectum is encompassed. The lateral borders of the PA field should include 1.5 to 2 cm margin beyond the pelvic brim, with appropriate blocking of almost all of the femoral head. Lateral fields should cover the anterior bony margin of sacrum with 1.5 to 2 cm margin posteriorly to allow for setup error and dosimetric coverage. Anteriorly, the field includes the internal iliac lymph nodes by placing its border on the posterior edge of pubic symphysis. Care is taken, however, when devising this border to ensure at least 3 cm coverage of the primary tumor anteriorly. In the superior-anterior portion of the field, it is usually possible to block a portion of small bowel. Similarly, the anterior genitalia in most patients should be blocked in the lateral fields. Custom boost fields are devised that include the GTV (or tumor bed) with a 2- to 3 cm margin. A three-field technique or laterals alone will often suffice.
Traditional field orientations (PA and laterals) are usually adequate for three-dimensional reconstructed targets. Although similar in shape to traditionally simulated fields, field shaping (blocking) can be more precisely individualized based on a CT-defined CTV. After CTV expansion of at least 1 cm to create a PTV (without IGRT), field borders are generally expanded another 5 to 10 mm in order to achieve sufficient dosimetric target coverage.
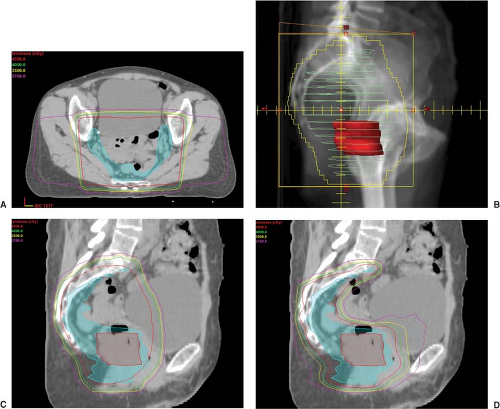
Possible benefits of treatment of rectal cancer with IMRT include dose escalation to the GTV and construction of a concave dose distribution that reduces small-bowel dose, in addition to bladder and bone marrow (see Fig. 24.4). Dosimetric studies comparing IMRT to traditional techniques in rectal cancer have shown clinically significant reductions in dose to bowel (80,81) as well as
bladder and femoral heads (82). The successful implementation of IMRT in the management of rectal cancer has been reported from multiple institutions generally with dose escalation to the GTV by and large with favorable outcomes (83,84,85). A retrospective review of 92 patients treated at the Mayo Clinic in Arizona demonstrated a 32% incidence of ≥ grade 2 gastrointestinal toxicity among patients treated with IMRT compared to 62% (p = 0.006) among patients treated with conventional fields during the same era (86). In view of the challenges of internal movement and distensibility pelvic organs (rectum, bowel, and bladder) as well as the dose inhomogeneity produced by some treatment planning systems, caution should be used before undertaking IMRT in rectal cancer.
bladder and femoral heads (82). The successful implementation of IMRT in the management of rectal cancer has been reported from multiple institutions generally with dose escalation to the GTV by and large with favorable outcomes (83,84,85). A retrospective review of 92 patients treated at the Mayo Clinic in Arizona demonstrated a 32% incidence of ≥ grade 2 gastrointestinal toxicity among patients treated with IMRT compared to 62% (p = 0.006) among patients treated with conventional fields during the same era (86). In view of the challenges of internal movement and distensibility pelvic organs (rectum, bowel, and bladder) as well as the dose inhomogeneity produced by some treatment planning systems, caution should be used before undertaking IMRT in rectal cancer.
Chemotherapy
Adjuvant or neoadjuvant radiotherapy is generally delivered with concurrent 5-FU. In the postoperative setting, protracted venous infusion (PVI) of 5-FU (225 mg/m2/day) throughout the course of pelvic radiotherapy has been shown to decreased tumor recurrence and improve overall survival compared to the administration of bolus 5-FU and is the current standard of care (79). In contrast, Intergroup 0144 subsequently showed no significant difference in outcome between PVI regimens and bolus 5-FU with leucovorin during radiotherapy (avoiding the need for central catheterization) (87). Multiple studies have reported encouraging results with the use of capecitabine, an orally administered premetabolite of 5-FU, in conjunction with pelvic radiation for rectal cancer (88,89,90,91). NSABP R-04 is currently investigating its use in comparison to PVI of 5-FU during preoperative radiotherapy.
Treatment Planning for Colon Cancer
Patients treated with radiotherapy for colon cancer are treated with the same chemotherapeutic and radiation principles as rectal cancer. The initial CTV generally includes the tumor bed and adjacent pelvic/retroperitoneal
lymphatics, with a subsequent boost to the tumor bed (or tumor preoperatively). Review of preoperative imaging, operative reports, and identification of surgical clips demarcating the tumor bed are essential in order to define targets and design fields that may impact local control and the prognosis of this disease.
lymphatics, with a subsequent boost to the tumor bed (or tumor preoperatively). Review of preoperative imaging, operative reports, and identification of surgical clips demarcating the tumor bed are essential in order to define targets and design fields that may impact local control and the prognosis of this disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree