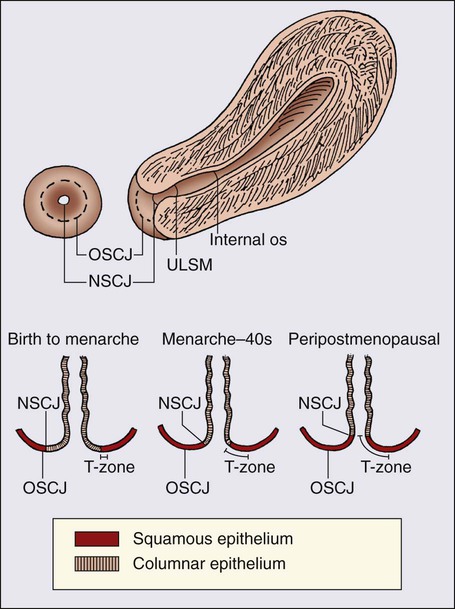
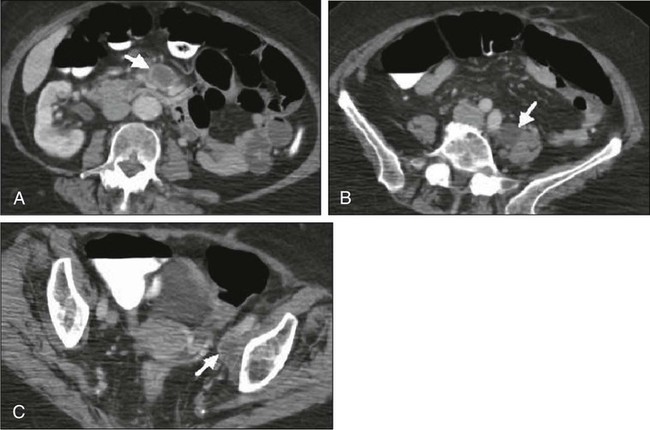
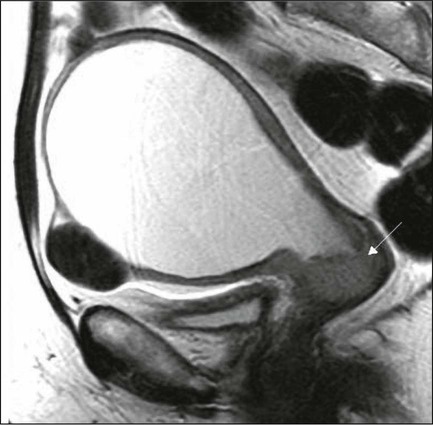
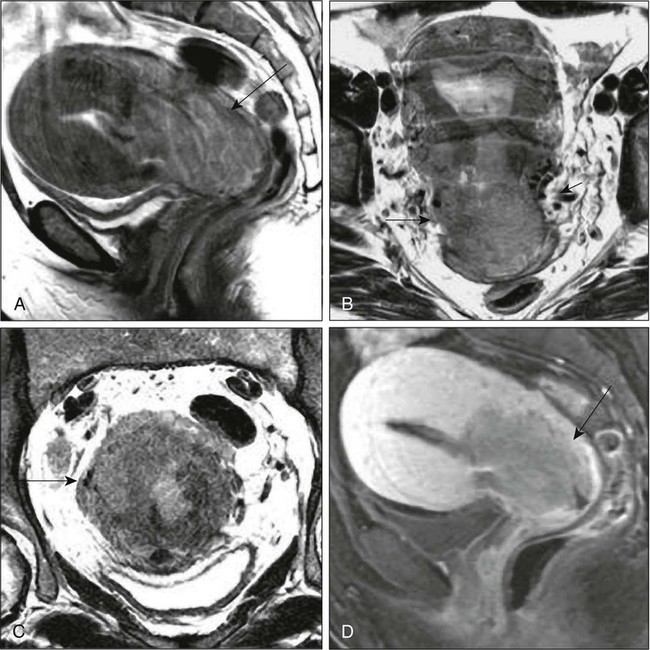
87 Anuja Jhingran, Anthony H. Russell, Michael V. Seiden, Linda R. Duska, Anne Kathryn Goodman, Susanna I. Lee, Subba R. Digumarthy and Arlan F. Fuller, Jr. Although cervical cancer is the third most common gynecologic malignancy in the United States, it ranks as the most common gynecologic malignancy worldwide and is the third most common cancer in women in the world, with an estimated 530,000 cases in 2008. Marked disparity in incidence exists between countries where routine gynecologic care and Pap smear screening are available, and countries in Latin America, the Caribbean, and Africa, where cervical cancer is the most common cause of cancer-related death in women.1 Molecular epidemiological studies have demonstrated that correlation of sexual activity with cervical carcinoma is related to transmission of the epithelial trophic and oncogenic HPV.4–4 Most HPV infections are transient, resulting in no changes or low-grade intraepithelial lesions (cervical intraepithelial neoplasia [CIN] 1) that will be spontaneously cleared in most young women.5,6 Development of high-grade intraepithelial lesions will occur in a small minority of women, usually within 24 months.7 High-grade lesions (CIN 2/3) may progress to invasive cervical cancer if not treated.8,9 Cases of CIN 2/3 that progress to invasive cancer will do so over a period of 8 to 12 years, which has been referred to as the detectable preclinical phase.10–13 Thus the opportunities for early detection and intervention are abundant. However, an estimated one half of the invasive cervical cancers diagnosed in the United States are found in women who have never been screened, and an additional 10% are diagnosed in women who have not been screened within the preceding 5 years.14 Based on a comprehensive review of available evidence, the American Cancer Society released new screening recommendations for the prevention and early detection of cervical cancer on March 12, 2012. Table 87-1 is a synopsis of their recommendations.15 The biggest change from the past is that Pap tests are no longer recommended every year and there are two types of test that are used for cervical cancer screening. The Pap test can find early cell changes and treat them before they become cancer. The HPV test finds certain infections that can lead to cell changes and cancer. The HPV test may be used along with a Pap test or to help doctors decide how to treat women who have an abnormal Pap test. Table 87-1 American Cancer Society Guidelines for Screening by Cytology for the Early Detection of Cervical Neoplasia and Cancer HPV is a double-stranded DNA virus in the Papovaviridae family (Fig. 87-1). This virus, made up of approximately 8000 nucleotides, encodes seven early genes and two late genes, as well as having a small, untranslated region. Approximately 100 different serotypes with limited DNA homology have been identified. Although the identification of high-risk subtypes of HPV has been important in defining potential therapeutic targets for the prevention of cervical carcinoma, HPV infection has not adequately explained all of the biological features of preinvasive disease and progression to invasive disease. Studies in sexually active college women demonstrated that infection with HPV is extremely common, occurring in up to 50% of women who become sexually active between the ages of 16 and 21 years, with the first abnormal Pap smear appearing in only a subset of women 1 year after infection. These infections typically are associated with low-grade dysplastic lesions and usually are transient. Persistent infection with associated high-grade dysplastic lesions is seen in only a small proportion of infected women (perhaps 1% or 2%). Biological and/or immunologic cofactors that allow the persistence of HPV infection in a small subset of women remain unclear. Epidemiological studies suggest that coinfection with herpes simplex virus type II, long-term oral contraceptive use, cigarette smoking, and high parity may increase the risk of persistent infection, carcinoma in situ (CIS), and invasive disease.16,17 Although long-term epidemiological studies undertaken since appreciation of the role of HPV remain incomplete, it is known that in the large majority of women, the period between HPV infection, dysplasia, and then invasive carcinoma typically is years to decades, offering the potential for screening and early intervention to change the natural history and morbidity associated with this disease.18–22 Molecular epidemiological studies have divided HPV serotypes into high-, intermediate-, and low-risk subtypes for the development of cervical neoplasia.23 Low-risk subtypes are associated with venereal warts (condylomata acuminata), whereas intermediate- and high-risk subtypes are associated with cervical dysplasia and invasive carcinoma. Recent worldwide review of HPV typing demonstrated that 87% of squamous cell carcinomas had an identifiable HPV genome associated with the tumor as compared with 76.4% of adenocarcinomas. HPV-16 was the predominant type, associated with between 46% and 63% of the squamous carcinomas, whereas HPV-18 was associated with 10% to 14% of squamous cell carcinomas. Sixteen other HPV types were associated with the remaining 25% of cases, including HPV-45, -31, and -33. Most epidemiological studies have demonstrated a higher incidence of HPV-18 (37% to 41%), followed by HPV-16 (26% to 36%) in women with adenocarcinoma of the cervix. Several authors have shown a positive association between the presence of certain HPV subtypes and prognosis. Barnes and colleagues24 showed that, among invasive carcinomas, HPV-18 is associated with poorly differentiated histology and higher incidence of nodal metastases. Similarly, Walker25 reported that HPV-18–associated cancers were more likely to recur than were HPV-16–associated cancers. By contrast, HPV-16 was associated with large cell-keratinizing tumors, and these tumors were less likely to recur.26 Lombard27 demonstrated HPV-18–associated tumors to have a relative risk of death 2.4 times greater than that observed for patients with HPV-16–associated tumors and 4.4 times greater than that for patients with a tumor associated with another HPV type. In a recent randomized, placebo-controlled study, the delivery of three vaccinations at day 0, month 2, and month 6 reduced the risk of persistent new HPV-16 infection from 3.8 cases per 100 women-years at risk to 0 per 100 woman-years in the group that were vaccinated, for a 100% efficacy rate. In this study, involving 2392 young women between the ages of 16 and 23 years, nine cases of HPV-16–related CIN were noted, all in the placebo recipients.28 The HPV vaccine with just HPV-16 and another double-variant vaccine are now commercially available and are recommended for both girls and boys from the ages of 9 through 31 years. The use of the vaccine worldwide should help eliminate cervical and head and neck cancers caused by HPV-16. Maiman, Fruchter, and associates29–32 investigated the disease characteristics, recurrence risks, and survival rates of human immunodeficiency virus (HIV)-seropositive patients with CIN and invasive cervical cancer. HIV-infected women had significantly higher rates of recurrence of CIN after standard therapies than did seronegative women. HIV-infected women with cervical cancer had significantly more advanced disease than did those who were not infected. Only 3 (19%) of 16 HIV-seropositive patients were first seen with an early-stage disease (defined as stage Ia or nonbulky Ib) compared with 35 (52%) of 68 in the HIV-seronegative group. When upstaged based on surgicopathological findings, only one (6%) HIV-infected patient had early-stage disease compared with 40% of uninfected patients. The response to therapy and prognosis was poorer among HIV-seropositive women, with higher recurrence and death rates. The majority of seropositive women had lymph node metastases and high-grade tumors. They generally were asymptomatic with respect to their HIV disease, but died of cervical cancer. The significant impact of immune status on disease progression was made evident by prolonged disease-free follow-up in seropositive patients with CD4 counts greater than 500/mm3, in contrast to those with CD4 counts less than 500/mm3. The observed marked increased risk of invasive cervical carcinoma in women infected with the HIV virus and the demonstration that highly effective antiretroviral therapy is capable of doubling the CIN regression rates in women infected with HIV, as compared with those women not receiving highly effective antiretroviral therapy, provides suggestive, indirect clinical evidence supporting the critical importance of the intact immune system in limiting the progression of HPV infection to invasive cancer in healthier populations. The epithelium of the cervix is composed of squamous epithelium that covers the exocervix and glands and columnar epithelial cells that line the endocervix. The border between the squamous and columnar epithelium is called the squamocolumnar junction, the site of ongoing squamous metaplasia believed to be most vulnerable to viral neoplastic transformation. With increasing age, the squamocolumnar junction migrates from the exocervix into the distal endocervical canal (Fig. 87-2), with the region between the original and subsequent locations termed the transformation zone. The transformation zone is the most common location for detection of early cervical cancers (Fig. 87-3). Tumors arising on the ectocervix typically are squamous cell carcinomas, whereas adenocarcinomas are more likely to have their epicenter in the endocervix. A continuum appears to exist from CIN to frankly invasive squamous cell carcinoma (Fig. 87-4). The mean age of women with CIN is 15.6 years younger than that of women with invasive cancer, suggesting slow progression of CIN to invasive carcinoma.18 The natural history of HPV infection and CIN in part reflects the host immune system response to the virus. Seventy-five percent of CIN 1 lesions will spontaneously regress or persist as CIN 1, without progression to invasive carcinoma.18–21 Miller22 reported, in a 13-year observational study, that only 14% of CIN 3 lesions had progressed, whereas 61% persisted, and the remainder disappeared. Patients taking corticosteroids or other immunosuppressive drugs and patients with HIV infection are at higher risk of progressing to invasive cancer and may have a shorter transit time for this progression. Approximately 75% of invasive cervical carcinomas are squamous cell carcinomas. Tumor histology differs between well, moderately, or poorly differentiated tumors. Squamous carcinomas (Fig. 87-5) may be keratinizing (sometimes containing characteristic keratin pearls) or nonkeratinizing. Large cell and small cell variants exist. True verrucous cancers of the cervix are rare. Clinically important subtypes also include clear cell adenocarcinomas of the cervix associated with in utero diethylstilbestrol (DES) exposure, which tend to be diagnosed at a younger age than most other adenocarcinomas, and so-called adenomalignum or minimal deviation adenocarcinoma (Fig. 87-6), an entity associated with deceptively bland or benign-appearing cells, which may be cause for undertreatment of a true malignancy, with a significant likelihood of recurrence even when diagnosed at an early stage.33,34 Adenosquamous carcinomas consist of a malignant glandular component and a malignant squamous component and make up approximately one-third of cervical carcinomas with glandular differentiation. Opinions vary regarding the prognosis of adenosquamous carcinoma compared with pure adenocarcinoma or pure squamous carcinoma when prognosis is adjusted for clinical stage at diagnosis. A clinically important variant of adenosquamous carcinoma is the so-called glassy cell carcinoma, thought to represent a very poorly differentiated adenosquamous carcinoma, the name of which derives from the ground-glass or granular appearance of the cytoplasm seen in many cases. Additional features may include an intense stromal inflammatory infiltrate composed predominantly of eosinophils and plasma cells. Some patients may have accompanying eosinophilia in their circulating blood, with elevated absolute eosinophil counts. This histologic type is associated with a rapid clinical rate of growth, proclivity for early regional dissemination, and increased risk of recurrence after surgical therapy or radiation therapy, even in the absence of other recognized adverse prognostic factors.35–38 Neuroendocrine tumors in the cervix include typical and atypical carcinoid tumors, and large cell, small cell neuroendocrine carcinomas, and undifferentiated small cell carcinoma. Both large cell and small cell neuroendocrine tumors resemble similar carcinomas arising in the lung and other aerodigestive sites.39,40 Undifferentiated small cell carcinoma is a very poorly differentiated carcinoma with neuroendocrine features, histologically similar to anaplastic small cell carcinoma of the lung.41 Neuroendocrine carcinomas tend to behave very aggressively, with frequent widespread metastasis to multiple sites, including bone, liver, and skin. Brain metastases may occur when disease is advanced but usually are preceded by lung metastases.43 Efforts to treat these tumors by using approaches typically used for small cell carcinomas of the lung have had mixed results.42 Screening for cervical cancer and its precursors with the Pap smear and pelvic examination has resulted in dramatic reductions in cervical cancer mortality in every country where this has been widely used, and is arguably the most effective screening program in effect for any neoplastic disease, in either gender. Table 87-1 outlines the current screening guidelines of the American Cancer Society. The false-negative rate of the Pap smear is approximately 10% to 15% in women with invasive cancer, but the sensitivity, as defined by the detection of biopsy-proven CIN, is 51%.45–45 The sensitivity of the test may be improved by ensuring adequate sampling of the squamocolumnar junction and the endocervical canal. Smears without endocervical or metaplastic cells may be inadequate and possibly should be repeated.46 In the 1980s, cytology laboratories began reporting an increasing number of smears with changes of “squamous atypia” in response to concerns from clinicians over an unacceptably high false-negative cytology rate and increased recognition by cytopathologists of the cytologic changes associated with HPV infection. The use of multiple classification systems with inconsistently defined numeric grading conventions added further imprecision. In an attempt to eliminate confusion among clinicians and cytopathologists, a uniform system for reporting epithelial cell abnormalities was established in 1988 at a National Cancer Institute (NCI) workshop for reporting cervical and vaginal cytologic diagnoses.47 The Bethesda System has since been revised, in 1991 and again in 2001 (Table 87-2),48 to reflect laboratory and clinical experience gained since the original implementation, as well as the increased use of new technologies and results from interval research studies. An important contribution of the Bethesda System was the creation of a standardized format and nomenclature for cytology laboratory reports that includes both a descriptive diagnosis and an evaluation of specimen adequacy. Table 87-2 • Atypical squamous cells (ASC) of undetermined significance (ASC-US), cannot exclude high-grade squamous intraepithelial lesion (HSIL [ASC-H]) • Low-grade squamous intraepithelial lesion (LSIL) encompassing human papillomavirus, mild dysplasia, cervical intraepithelial neoplasia (CIN) 1 • HSIL encompassing moderate and severe dysplasia, carcinoma in situ; CIN 2 and CIN 3 Adapted from Soloman D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002;287:2114. Management of the patient with an abnormal Pap smear with respect to further diagnostic assessments, therapeutic intervention, and subsequent surveillance follow-up is a complex arena in which guidelines continue to evolve. The American Society for Colposcopy and Cervical Pathology (ASCCP) has developed contemporary guidelines for management of cervical cytologic abnormalities and CIN based on current reporting terminology (Bethesda 2001).49,50 Techniques for further evaluation can include colposcopy, endocervical curettage or brushing, and cervical conization by cold knife or loop electrodiathermy excision procedure (LEEP). Testing for high-risk, oncogenic HPV DNA51–57 synchronously, or subsequent to cytologic screening, is proving to be a useful complementary tool in triage of patients with ASC-US smears, and in determining the need for colposcopy and the intervals for repeated screening. It may be helpful in the follow-up of younger patients with LSIL smears, and a useful tool in identifying older women who can safely be screened at 3-year intervals instead of annually. HPV triage of patients with an ASC-US smear is at least as sensitive as immediate colposcopy for ultimately detecting CIN 3 and results in referral of about half as many women to colposcopy. A follow-up strategy that uses repeated cytology is sensitive at an ASC-US referral threshold, but requires two follow-up visits and, ultimately, more colposcopic examinations than does HPV triage.58 Patients with a gross lesion of the cervix should undergo cervical biopsy. For patients with an abnormal cytologic evaluation, without a gross lesion, a colposcopic examination with directed punch biopsies is required. Colposcopy allows the clinician to identify areas suggestive of dysplasia. A 3% acetic acid solution is applied to the cervix for 30 to 90 seconds, which causes a transient reaction with the envelope proteins of the papillomavirus, in addition to producing an osmotic dehydration of the dysplastic cells, thereby accentuating the optically dense chromatin to produce a whitish area. The skilled colposcopist can further distinguish between grades of dysplasia based on acetowhitening and types of vascular patterns (Fig. 87-7). An additional technique to help visualize abnormal areas is the application of quarter-strength Lugol iodine after the initial inspection for acetowhitening. High-grade lesions turn mustard yellow. Study of the endocervical canal is required when no abnormalities are found on colposcopic examination, when the entire squamocolumnar junction cannot be visualized, or when atypical endocervical cells are present on Pap smear. Some experts advocate the use of endocervical curettage (ECC) as part of every colposcopic examination to safeguard against missing occult cancer within the endocervical canal. Others reserve ECC for patients with recurrent cytologic atypia after therapy. A sleeved endocervical brush is an alternative to ECC that is less uncomfortable for many patients. A recent, prospective comparison of ECC specimens with sleeved endocervical brush specimens (both obtained from the same patient sequentially after randomization with respect to the order of obtaining specimens) before cervical conization or hysterectomy revealed a higher rate of inadequate specimens from ECC, comparability of the two techniques with respect to sensitivity and specificity in unmatched analysis, and superior sensitivity of the sleeved endocervical brush in matched analysis. As the sleeved endocervical brush is at least isoeffective and possibly better than ECC but more comfortable for the patient, many clinicians prefer this maneuver, which may serve to increase patient compliance with subsequent surveillance follow-up and repeated assessment.59 LEEP uses wire-loop electrodes in conjunction with a radiofrequency alternating current to excise the entire transformation zone and distal canal under local anesthesia (Fig. 87-8). Compared with ablative procedures, LEEP has the major advantage of obtaining tissue for histologic evaluation. At many centers, LEEP has become the preferred treatment for CIN that can be assessed adequately with colposcopy.60–72 Complications include bleeding, with a reported incidence of 1% to 8%,73,74 cervical stenosis (1%), and, rarely, pelvic cellulitis or adnexal abscess. In some cases, LEEP may not be an adequate alternative to formal excisional conization, such as in those patients in whom microinvasive or invasive cancer is suspected, or in those with adenocarcinoma in situ, because it may treat disease within the cervical canal inadequately and complicate pathological interpretation of the specimen. Appropriate application of this technique will yield tissue suitable for pathological study and reliable diagnosis. Excess heat may result in thermal artifact that can compromise interpretation of margins and the therapeutic adequacy of the LEEP procedure. Diagnostic or therapeutic excisional conization (cone biopsy) must be performed under general or regional anesthesia. Complications, which include hemorrhage, sepsis, infertility, stenosis, and cervical incompetence, occur in 2% to 12% of patients, depending on depth and geometry of excision.75–75 Width and depth of the cone should be tailored to the topography of the lesion, to produce the least amount of injury while providing clear surgical margins. Conization may be performed with a cold knife or with the carbon dioxide laser. The revised 2009 staging criteria (Table 87-3)76 of the International Federation of Obstetrics and Gynecology (FIGO) are a mixture of histopathological, clinical, and radiographic assessments that reflect the fact that invasive cervical cancer is most prevalent in less-developed portions of the globe where sophisticated and expensive imaging modalities may not be widely available. Cervical cancer is clinically staged, and staging is based primarily on inspection and palpation of the cervix, vagina, parametrium, and pelvic sidewalls. Only the subclassification of stage I (Ia1, Ia2) requires pathological assessment. The FIGO staging system permits assessment through biopsy, physical examination, cystoscopy, proctoscopy, excretory urography (intravenous pyelography), and plain film radiography of the chest and skeletal system. Results of lymphangiography, computed tomography (CT), MRI, and positron emission tomography (PET) may be of great value in planning treatment, but do not influence assignment of clinical stage in the FIGO formalism. When findings are equivocal, by convention, a patient is assigned to a lower stage. Once clinical stage has been assigned, it cannot be altered by subsequent events or findings. Findings from surgical evaluation (by laparoscopy or by surgical assessment of retroperitoneal lymph nodes via extraperitoneal or transperitoneal node dissection) will not alter assignment of clinical stage. However, these findings may profoundly influence subsequent treatment. Similarly, evidence of nodal or other spread discerned at the time of hysterectomy does not alter clinical stage. Table 87-3 FIGO 2009 Staging for Cervical Cancer The FIGO staging system is based predominantly on clinical examination under anesthesia, ultrasonography, intravenous urography, cystoscopy, proctoscopy, and chest radiography. Significant inaccuracies occur in this staging because of possible errors in gynecologic examination (24% to 39%).77 The various imaging modalities have a complementary role in the accurate staging and complete evaluation of the cancer that have important therapeutic implications. Today, clinicians most often obtain a contrast-enhanced CT scan of the abdomen and pelvis for patients with disease of stage Ib2 or greater (Fig. 87-9).78 Lymph nodes larger than 1.0 to 1.5 cm in diameter are suspicious for tumor involvement and should be biopsied. Unfortunately, microscopic metastases are not readily detected with CT, and the inflammation commonly associated with advanced disease may cause enlargement of nodes that do not contain metastases. The sensitivity of MRI in the detection of regional metastases is similar to that of CT. However, MRI provides better anatomic delineation and accurate estimation of the tumor size, volume, and local extent within the pelvis, which can influence the choice of therapy (Fig. 87-10). Involvement of the vagina, parametrium, pelvic wall muscles, ureter, bladder, and rectum can be better assessed with MRI for accurate staging (Fig. 87-11).77 MRI before and after vaginal opacification with contrast medium can be used if imaging evaluation of the vaginal wall or fornices is required.79 T2-weighted images obtained by using phased-array coil, fast spin-echo or conventional spin-echo techniques are accurate in local staging and lymph nodal assessment; the former technique is faster with increased resolution.80 Dynamic contrast-enhanced T1-weighted images are helpful in identifying smaller tumors, fistulous tracts, and invasion into bladder and rectum.77 Dynamic contrast-enhanced imaging is useful in differentiating areas composed predominantly of tumor cells (well-enhanced areas) from those of fibrous tissue with scattered cancer cells (poorly enhanced areas). This information can be helpful, as radiation therapy is more effective in well-enhanced tumors.81 MRI is very useful in the evaluation of tumor volume and enlarged lymph nodes, inherently important prognostic factors as well as determinants of the design of radiation treatment ports and selection of radiation dose. However, MRI cannot identify micrometastases to lymph nodes and differentiate malignant from nonmalignant enlargement. MRI with newer contrast agents like ultrasmall superparamagnetic iron oxide has been useful in distinguishing benign from malignant nodes.82 The shape, volume, and direction of the growth of the tumor can be well assessed, and these are crucial for planning brachytherapy and external beam radiotherapy.77 MRI also is useful in the follow-up evaluation of the tumor, its response to treatment, and identification of recurrences. However, benign conditions, like edema or inflammation, sometimes cannot be differentiated from tumors. Dynamic contrast-enhanced MRI is useful in differentiating malignant lesions that usually have shorter and stronger enhancement than benign conditions in patients who have abnormalities after treatment for cervical cancer.83 Recent studies have suggested that 18F-fluorodeoxyglucose PET (FDG-PET) is more sensitive and specific than more standard radiographic studies in the detection of lymph node involvement by cervical cancer.86–86 Grigsby and colleagues87 have reported a strong correlation between abnormal posttherapy FDG uptake and tumor recurrence. PET scans are now Medicare approved and considered standard of care for staging purposes for all newly diagnosed cervical carcinomas. Routine laboratory assessment should include complete blood counts with differential counts and red cell indices. Many patients with advanced disease will be anemic at the time of diagnosis, often reflecting chronic blood loss and iron deficiency. Anemic patients treated with radiation or chemoradiation have poorer outcomes than do patients with near-normal hemoglobin levels.88–91 Anemia at diagnosis (before treatment) correlates significantly with reduced pelvic control and survival with univariate analysis, but not always when data are subjected to multivariate analysis. In contrast, multivariate analysis reveals that hemoglobin level during the course of radiation therapy or chemoradiation is a robust predictor of local outcome and survival.88–91 Transfusion for anemic patients with maintenance of average weekly nadir hemoglobin levels at or above 11 to 12 g/dL through radiation therapy is associated with improvement in prognosis to that associated with patients with near-normal or normal hemoglobin levels at diagnosis.88,90 This effect may be partially mediated through better tumor oxygenation and oxygen-enhanced radiation lethality to clonogenic cells, as well as reduced angiogenesis in better-oxygenated tumors. With pelvic or extended-field radiation that will encompass a substantial percentage of adult bone marrow, hemoglobin may decline slowly, even when adequate iron stores are present and patients are supported with hematinics. Concurrent administration of cisplatin further aggravates this problem, and can result in clinically significant reduction in hemoglobin levels over the course of a 6- to 8-week program of chemoradiation, even in patients with normal hemoglobin levels at the time of diagnosis. Frequent monitoring of hemoglobin levels as well as white cell counts and platelets should be performed throughout a course of chemoradiation, and hemoglobin level should be supported, either by transfusion or through the use of recombinant erythropoietin. The minimal hemoglobin level required is uncertain, but it seems prudent to target a minimum of 10 g/dL. Neutropenia may indicate supportive treatment with granulocyte colony-stimulating factor to avoid prolonged treatment interruptions that are known to adversely affect local tumor control in patients treated with radiation.92,93 Absolute neutrophil counts should be monitored at least weekly in patients undergoing chemoradiation. An elevated platelet count has been associated with advanced malignancy and is considered a consequence of increased platelet production.94 Hernandez and associates95 identified this effect in advanced cervical cancer, and Rodriguez and coworkers96 identified the preoperative platelet count as an adverse prognostic factor, even for patients with stage Ib cervical carcinoma. The cumulative 5-year survival of women with a platelet count greater than 300,000 (85 women) was 65%, compared with 84% for the group with a normal value (134 women). At issue was the question of whether the value could have been elevated simply because of bleeding from the primary tumor, but no association was found with the preoperative hematocrit. This study of surgically treated patients with early disease also demonstrated that the effect was not a consequence of metastatic disease and did correlate with tumor volume, with nearly half of the patients with platelet counts in excess of 300,000 having “large” lesion size, in contrast to only 28% (32 of 114) patients with normal counts.97 In a multivariate analysis, adjusting for age, race, tumor size, and presence of lymph node metastases, high platelet count was still associated with an adverse prognosis. Prognostic factors fall into two groups: tumor-related factors and patient-related factors. Probably the most important tumor-related factor is tumor size, and this is true both for patients treated with hysterectomy97,98 and for patients treated with radiation therapy.101–101 In fact, FIGO, in 1995, divided stage Ib into two groups according to size; other stage categories act, in part, as surrogates for tumor size. For patients treated with surgery, histologic evidence of extracervical spread is associated with a poorer prognosis. Parametrial extension is associated with a higher rate of lymph node involvement, local recurrence and death from cancer.102,103 Uterine body involvement is associated with an increased rate of distant metastases in patients treated with radiation therapy or surgery.104,105 Lymph node involvement is another important tumor-related factor. After radical hysterectomy, reported-year survival rates usually are about 35% to 40% lower when the pelvic lymph nodes are involved.106,107 However, studies suggest that postoperative chemoradiation improves these results.108 Several reports suggest that survival decreases with increasing size of the largest involved nodes,109,110 increasing number of nodes involved,106,110,111 and the increasing level of regional involvement and with extent of central disease in the cervix. Overall, the survival rates for patients with positive paraaortic nodes are about half those of patients who have similar stages of disease without paraaortic lymph node involvment.114–114 Lymphovascular space invasion (LVSI) also is correlated with an increased risk of recurrence. This reflects, in part, the strong correlation between LVSI and lymph node involvement; however, in a large number of postoperative studies, LVSI is an independent predictor of prognosis.107,115–117 Investigators have compared the outcome of patients with adenocarcinomas with squamous carcinomas and have reached varying conclusions about the relative prognoses of the two histologic types of cervical cancer.120–120 In a review of 1767 patients with stage Ib disease (229 with adenocarcinoma), Eifel and colleagues121 found that patients with adenocarcinoma had a significantly higher risk of recurrence and death from disease, independent of age, tumor size, or tumor morphology. The rate of distant metastasis for patients with bulky (>4 cm) adenocarcinomas was almost twice that for patients with squamous carcinoma (37% vs. 21%, P < 0.01). Several investigators have reported high recurrence rates after radical hysterectomy for adenocarcinomas. In a subset analysis of a randomized Gynecologic Oncology Group study, Rotman and associates122 reported a high recurrence rate (11 of 25 patients [44%]) after treatment with radical hysterectomy alone for adeno-adenosquamous carcinomas of the cervix; in contrast, only 3 of 31 patients (10%) who received postoperative radiation therapy had recurrences. However, all of these comparisons had a relatively small number of patients treated with cervical adenocarcinomas. Other tumor factors include correlation between the serum concentration of squamous cell carcinoma antigen and the extent of squamous carcinoma of the cervix.125–125 Increased tumor vascularity has been associated with a relatively poor prognosis,126,127 and a strong inflammatory response in the cervical stroma tends to predict a good outcome.126 Some authors have reported a correlation between HPV subtype and prognosis.27 Several investigators have reported a higher recurrence rate in patients with histologically negative lymph nodes when a polymerase chain reaction assay of the lymph nodes was strongly positive for HPV DNA.128,129 Other authors have correlated poor prognosis with the presence of HPV messenger RNA128 or HPV-related proteins130 in the peripheral blood of cervical cancer patients. Patient-related factors have been discussed elsewhere in this chapter and include age, hemoglobin, platelet counts, race, and smoking.131 Several studies have demonstrated that in the United States, women of color with invasive cervical cancer present with higher-stage disease134–134 and lower hemoglobin levels135,136 than do white women. Differences in socioeconomic status may influence minority patients’ access to care and ability to comply with treatment recommendations.136 In a study of 1304 women treated with radiation for cervical cancer, Kucera and colleagues131 reported that smokers with cervical cancer had a poorer 5-year survival rate than nonsmokers. This difference was statistically significant for patients with stage III disease (5-year survival rate 20.3% vs. 33.9%, P < 0.01). However, the authors did not analyze the possible influence of smoking-related deaths from causes other than cervical cancer. Patients with large FIGO stage IIa or with stages IIb through IVa disease usually are managed with chemoradiation. Some patients with an incomplete response to chemoradiation may benefit from a combined approach using adjunctive hysterectomy to clear persistent central disease after chemoradiation. Patients with bulky Ib2 or IIa disease and rare patients with bulky central stage IIb lesions with minimal, medial parametrial invasion may be considered for this approach. For patients in whom disease recurs centrally in the pelvis after maximal chemoradiation, radical exenterative surgery can be performed, provided that no distant disease is present. Optimal candidates have mobile central disease without lymph node involvement.139–139 Intraoperative radiation therapy may be part of salvage surgery when lateral or posterior surgical margins will be predictably inadequate.140,141 Recurrent disease after initial radical surgery historically has been treated with salvage radiation, but currently should be treated with chemoradiation.142 Hysterectomy involves the removal of the uterus and varying amounts of surrounding tissue (Fig. 87-12). Because the risk of ovarian metastases is low (0.5%), ovarian preservation usually is recommended in premenopausal women, obviating the need for hormone replacement therapy.143 An important exception is when nodal metastases from a primary cervical adenocarcinoma are detected intraoperatively, when the risk of ovarian metastasis escalates steeply, perhaps to as high as 25%.144 Five types of hysterectomy have been described.145
Cancers of the Cervix, Vulva, and Vagina
Cervix Cancer
Epidemiology

Human Papillomavirus Biology
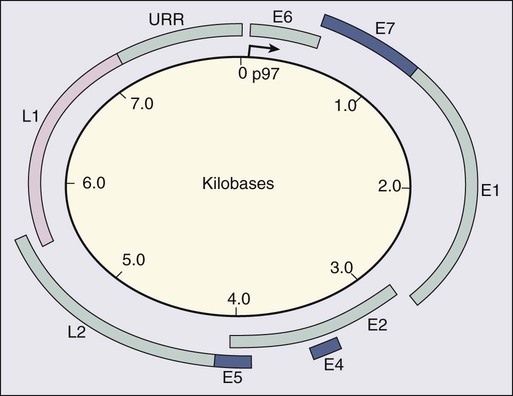
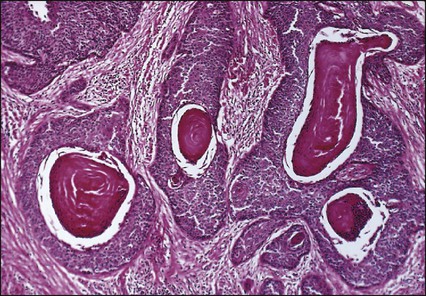
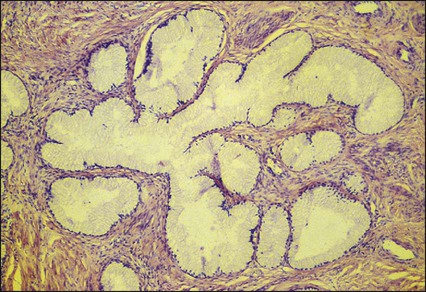
Pathology
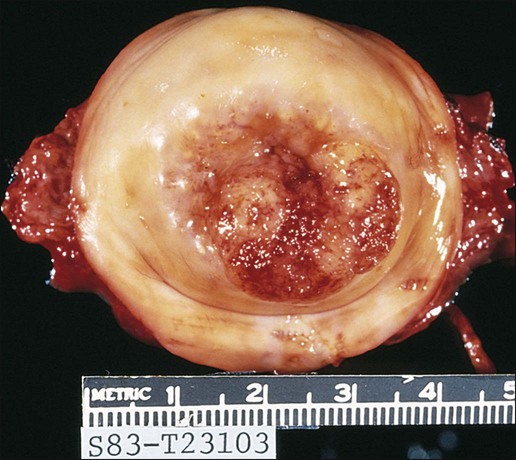
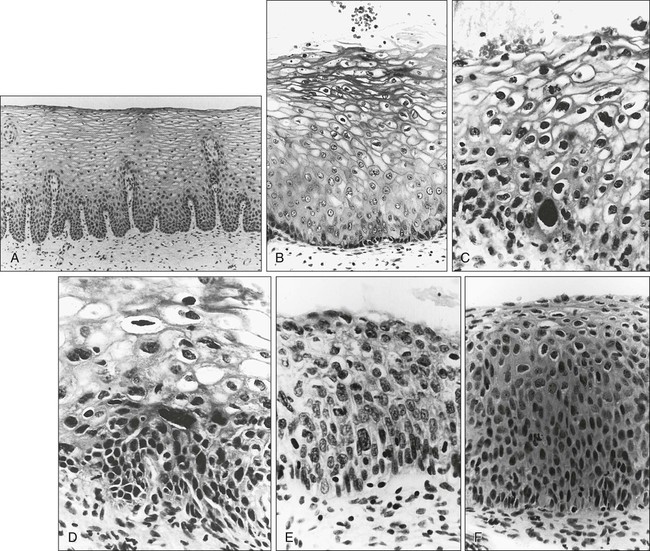
Squamous Cell Carcinomas of the Cervix
Cervical Adenocarcinomas
Adenosquamous Carcinomas
Neuroendocrine Tumors of the Cervix
Screening
SPECIMEN ADEQUACY
Satisfactory for evaluation (note presence of endocervical/transformation zone component)
Unsatisfactory for evaluation (specify reason)
Specimen rejected/not processed (specify reason)
Specimen processed and examined, but unsatisfactory for evaluation of epithelial abnormality because of (specify reason)
GENERAL CATEGORIZATION (OPTIONAL)
Negative for intraepithelial lesion or malignancy
Epithelial cell abnormality
Other
INTERPRETATION/RESULT
Negative for intraepithelial lesion or malignancy
Organisms
Other nonneoplastic findings (Optional. List not comprehensive)
Reactive cellular changes associated with inflammation (includes typical repair)
Glandular cells after hysterectomy
Atrophy
Epithelial cell abnormalities
Squamous cell
Glandular cell
Other (list not comprehensive)
AUTOMATED REVIEW AND ANCILLARY TESTING
(Include as appropriate)
EDUCATIONAL NOTES AND SUGGESTIONS
(Optional)
Diagnosis
Colposcopy
Endocervical Curettage or Endocervical Brush
Loop Electrodiathermy Excision Procedure
Diagnostic or Therapeutic Excisional Conization (Cone Biopsy)
Staging
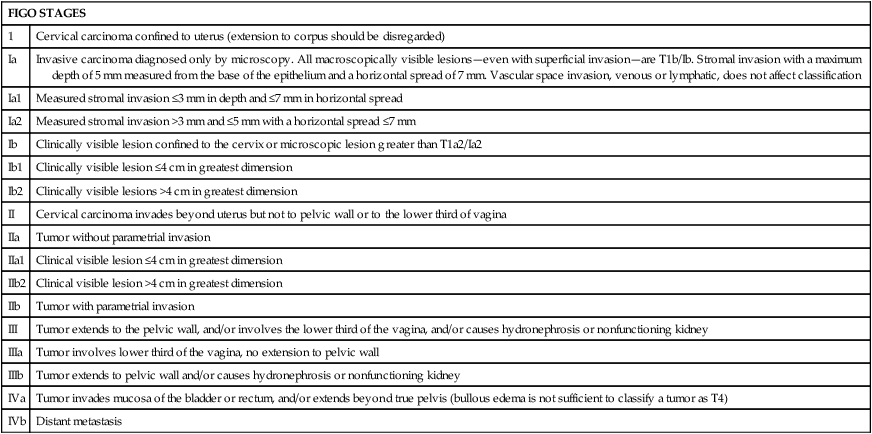
FIGO STAGES
1
Cervical carcinoma confined to uterus (extension to corpus should be disregarded)
Ia
Invasive carcinoma diagnosed only by microscopy. All macroscopically visible lesions—even with superficial invasion—are T1b/Ib. Stromal invasion with a maximum depth of 5 mm measured from the base of the epithelium and a horizontal spread of 7 mm. Vascular space invasion, venous or lymphatic, does not affect classification
Ia1
Measured stromal invasion ≤3 mm in depth and ≤7 mm in horizontal spread
Ia2
Measured stromal invasion >3 mm and ≤5 mm with a horizontal spread ≤7 mm
Ib
Clinically visible lesion confined to the cervix or microscopic lesion greater than T1a2/Ia2
Ib1
Clinically visible lesion ≤4 cm in greatest dimension
Ib2
Clinically visible lesions >4 cm in greatest dimension
II
Cervical carcinoma invades beyond uterus but not to pelvic wall or to the lower third of vagina
IIa
Tumor without parametrial invasion
IIa1
Clinical visible lesion ≤4 cm in greatest dimension
IIb2
Clinical visible lesion >4 cm in greatest dimension
IIb
Tumor with parametrial invasion
III
Tumor extends to the pelvic wall, and/or involves the lower third of the vagina, and/or causes hydronephrosis or nonfunctioning kidney
IIIa
Tumor involves lower third of the vagina, no extension to pelvic wall
IIIb
Tumor extends to pelvic wall and/or causes hydronephrosis or nonfunctioning kidney
IVa
Tumor invades mucosa of the bladder or rectum, and/or extends beyond true pelvis (bullous edema is not sufficient to classify a tumor as T4)
IVb
Distant metastasis
Diagnostic Imaging Evaluation of Cervical Cancer
Laboratory Evaluation
Prognostic Factors
Treatment Overview
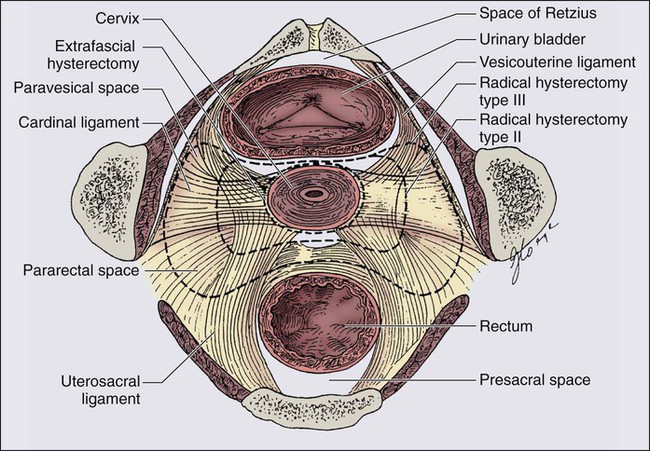
Hysterectomy
Cancers of the Cervix, Vulva, and Vagina