Cancers of the Central Nervous System
Lucien A. Nedzi
Kevin S. Choe
Arnold Pompos
Ezequiel Ramirez
Introduction
The central nervous system (CNS) comprises the brain, the spinal cord, and their coverings. Patients with benign lesions may live out their natural lifespan, whereas the survival of those with malignant tumors is frequently measured in weeks to months. The optimal radiation therapy treatment technique should maximize the therapeutic benefit and minimize the potential toxicities, especially for long-term survivors.
Natural History
Anatomy of the Brain
Knowledge of the basic topographic and functional anatomy of the brain is critical for tumor localization and delineation of regions that need to be spared during treatment. Generally, the brain consists of three major divisions: The cerebrum, cerebellum, and brainstem. The cerebral hemispheres and midline structures are supratentorial, and the cerebellum and lower brainstem are infratentorial. The longitudinal cerebral fissure divides the cerebrum into two hemispheres. Each hemisphere is divided by the major sulci into six lobes: Frontal, parietal, occipital and temporal, and the midline central and limbic lobes. The central sulcus (of Rolando) separates the frontal lobe from the parietal lobe. The parietal-occipital fissure separates the parietal lobe from the occipital lobe. The lateral fissure (of Sylvius) defines the temporal lobe boundaries. The cerebral hemispheres are connected by the corpus callosum, beneath which are located the midline structures (third ventricle, pineal body, and midbrain) and the deep paramedian structures (lateral ventricles, caudate nucleus, lentiform nucleus, thalamus, and hypothalamus).
There are three historic approaches to defining the functional anatomy of the cerebral hemispheres. Brodmann’s schema numbers 52 areas of structural specialization to provide both anatomic and functional “road map” of the brain by which tumor location can be described (1,2). Another approach is a regional (lobe) division of function. The occipital lobe is primarily involved with vision and its dependent functions. The temporal lobe processes sound, vestibular sensations, sights, smells, and other perceptions into complex “experiences” important for memory. Wernicke’s area is located on the posterior portion of the superior temporal gyrus and plays a critical role in receptive speech. The parietal lobe, specifically the postcentral gyrus, is involved in somatosensory function, sensory integration (body image), and Gnostic (perceptive) functions. The frontal lobe is associated with higher level cognitive functions, such as reasoning and judgment. The frontal lobe contains the primary motor cortex (precentral gyrus) and Broca’s area (inferior third of the frontal gyrus), which is important in expressive speech. The limbic lobe mediates memories, drives, and stimuli. It affects visceral functions central to emotional expression, including sexual drive. Finally, the central lobe (insula) is important in visceral sensation and motility. The most modern method to describe functional neuroanatomy is through various techniques of functional mapping. Classic mapping utilizes microelectrode stimulation of the cortical surface directly. Newer noninvasive techniques such as functional magnetic resonance imaging (fMRI), positron emission tomography (PET), and magnetoencephalography (MEG) are increasingly being integrated into clinical practice (3,4,5). Together, these procedures allow for precise mapping of function in an individual and can accurately predict deficits related to injury of a given area by tumor or therapy.
Epidemiology of Primary Central Nervous System Tumors
Annually, an estimated 63,000 new cases of primary nonmalignant and malignant CNS tumors are diagnosed in the United States with an estimated 13,000 deaths (6,7). The incidence of all primary nonmalignant and malignant brain and CNS tumors is 18.71 cases per 100,000 person-years. In the United States, the rate is slightly higher in females (19.88 per 100,000 person-years) than males (17.44 per 100,000 person-years) (7). The incidence rates are higher in more developed countries than in less-developed countries (8). The incidence rate of childhood primary nonmalignant and malignant brain and CNS tumors is 4.7 cases per 100,000 person-years. The rate is (4.75 per 100,000 person-years in males and 4.66 per 100,000 person-years in females) (7).
Most of the primary CNS tumors are located within the frontal, temporal, parietal, and occipital lobes of the brain.
Sixty-one percent of gliomas occur in the frontal, temporal, parietal, and occipital lobes. Tumors in other locations in the cerebrum account for another 3%. Of all tumors, 2%, 4%, and 2% are found in the ventricles, cerebellum, and brainstem, respectively. The pituitary and pineal gland account for ∼7% of tumors. Tumors of the meninges represent 24% of all tumors (7).
Sixty-one percent of gliomas occur in the frontal, temporal, parietal, and occipital lobes. Tumors in other locations in the cerebrum account for another 3%. Of all tumors, 2%, 4%, and 2% are found in the ventricles, cerebellum, and brainstem, respectively. The pituitary and pineal gland account for ∼7% of tumors. Tumors of the meninges represent 24% of all tumors (7).
The overall incidence of primary spinal cord tumors is approximately 10% to 19% of all primary brain tumors (9). Schwannomas and meningiomas account for ∼60% of primary spinal tumors, with schwannomas being slightly more frequent; both types occur primarily in adults. Most primary spinal gliomas are ependymomas with a predilection for the cauda equina. The frequency of individual spinal cord tumors is quite different from that of their histopathologic counterparts in the brain. Gliomas constitute 46% of primary intracranial tumors but only 23% of spinal tumors. The incidence ratios of intracranial to intraspinal astrocytomas, ependymomas, and meningiomas are approximately 10:1, 3:1, and 18:1, respectively (10). Finally, the incidence ratio of intracranial to intraspinal tumors is higher up to four times in pediatric patients than in adults.
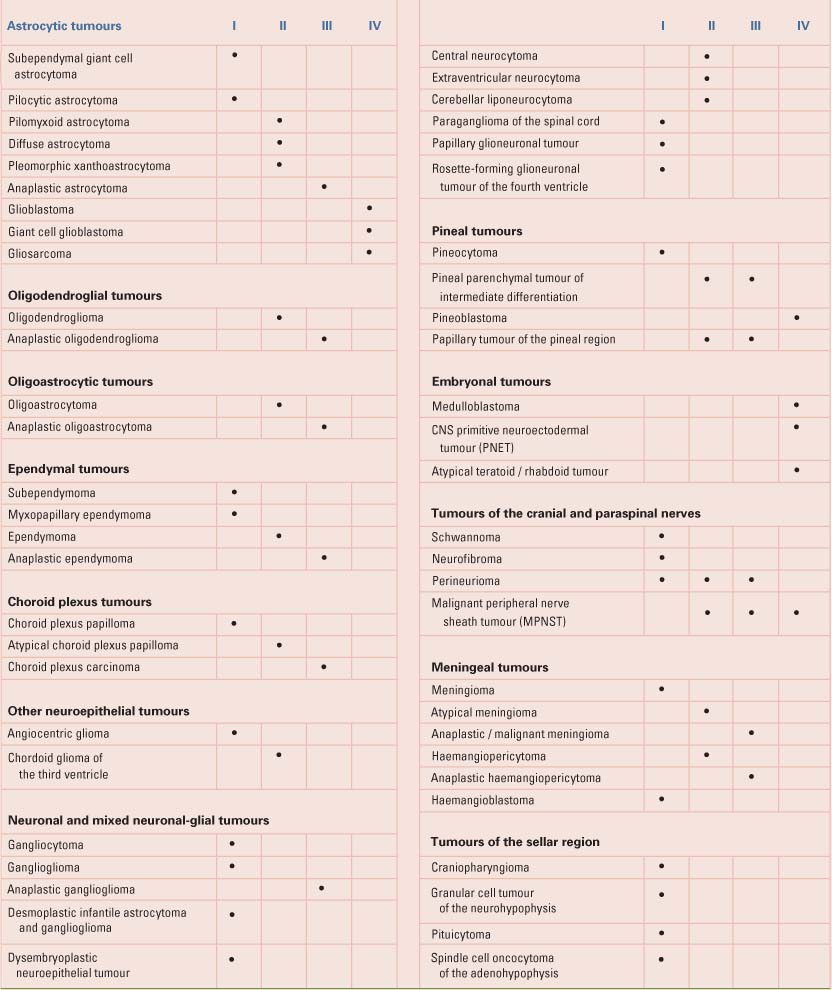
Table 31.1 shows the current WHO pathologic classification system of common primary CNS tumors (11). The most frequently reported histology is meningioma, which accounts for over 29% of all tumors, followed closely by glioblastoma and astrocytoma. The predominately benign nerve sheath tumors account for 8% of all tumors, of which 54% are acoustic neuromas. Pituitary tumors compose 6% of these lesions. Tumors of the glial cell origin represent about a half of all tumors. These include astrocytomas, glioblastomas, oligodendrocytomas, ependymomas, mixed gliomas, and neuroepithelial tumors. Astrocytomas and glioblastomas account for three-quarters of gliomas—the majority being glioblastomas (7). The most common spinal cord intramedullary tumors are those that are derived from glial precursors (astrocytes, ependymocytes, and oligodendrocytes) (12).
Epidemiology of Tumors Metastatic to the Central Nervous System
Metastatic brain tumors are the most common intracranial neoplasms in adults and it is about 10 times more common than primary intracranial tumors. In two cohorts of patients diagnosed with colorectal, lung, breast, or kidney carcinoma or melanoma, brain metastases were diagnosed in 8.5% to 9.6% (13,14). The cumulative incidence was estimated between 16% and 20% in patients with lung carcinoma, 7% in patients with renal carcinoma, 7% in patients with melanoma, 5% in patients with breast carcinoma, and 1% to 2% in patients with colorectal carcinoma.
With the exception of a primary paraspinal or neuraxis tumor, spinal cord tumors occur most often in the setting of disseminated disease from a distant primary tumor site. The spine is overall the most common site of bony metastases, with a reported incidence of 40% in patients with cancer (15). Of those patients with spine metastases, 10% to 20% develop malignant spinal cord compression (MSCC), accounting for 14,100 to 28,200 cases annually (16,17,18). MSCC from epidural metastases occurs in 5% to 10% of all patients with cancer and in up to 40% of patients with preexisting nonspinal bone metastases (15,19,20,21). MSCC may involve the spinal cord at any level, and symptoms depend on the location of the compression. The incidence of MSCC by vertebral levels is 10% to 16% cervical, 35% to 40% in T1 to 6, 44% to 55% in T7 to 12, and 20% in the lumbar spine (22,23,24). In 10% to 38% of cases, metastatic lesions present initially at multiple, noncontiguous levels (22,25,26).
The histology of MSCC follows the incidence patterns of primary malignancies, with the most common histologic diagnoses (i.e., breast, lung, and prostate) accounting for approximately half of all cases (6,15). Approximately 25% of all patients with MSCC have breast cancer, 15% have lung cancer, and 10% have prostate carcinomas. Overall, 5.5% of patients with breast cancer, 2.6% of patients with lung cancer, 7.2% of patients with prostate cancer, and 0.8% of patients with colorectal cancer experience a MSCC (17). Other commonly reported histologic diagnoses in adults include, in the order of cumulative incidence, multiple myeloma, nasopharynx, renal cell, melanoma, small cell lung, lymphoma, and cervix (15,17,27).
Workup and Staging
For both benign and malignant CNS tumors, magnetic resonance imaging (MRI) is the gold standard for imaging (28). The preferred slice thickness of MRI is ≤5 mm with ≤2.5 mm slice sampling. T1-weighted images with contrast provide excellent visualization of contrast-enhancing tumors, such as meningiomas, glioblastoma multiforme, and brain metastases. T2-weighted images generally demonstrate areas of edema, and T1-weighted fluid-attenuated inversion recovery (FLAIR) images better delineate infiltration by low- or high-grade gliomas. MRI registration with the treatment-planning computed tomography (CT) scan is therefore essential for target delineation. Additional imaging studies can reflect the biologic characteristics of CNS tumors, such as tumor metabolism, proliferation, oxygenation, blood flow, and the function of surrounding normal brain; these include MRI spectroscopy, fMRI, PET scans, and single photon emission tomography (SPECT) scans (29,30,31). After radiation therapy, PET scans and MRI spectroscopy assist in differentiating active tumor versus radionecrosis.
MRI of the entire neural axis along with cerebrospinal fluid (CSF) cytology is required for staging of tumors with a high propensity for spread within the CNS by involvement of the CSF, leptomeninges, or spinal cord. These tumors include medulloblastomas, primitive neuroectodermal tumors (PNETs), anaplastic ependymomas, choroid
plexus carcinomas, pineoblastomas, germ cell tumors, and lymphomas.
plexus carcinomas, pineoblastomas, germ cell tumors, and lymphomas.
Table 31.1 WHO Grades of CNS Tumours | |
|---|---|
|
In patients who present for urgent symptom management, CT scan can be obtained rapidly, providing information on ventricular obstruction, hemorrhage, or edema. Owing to the risk of herniation and death, lumbar puncture should be avoided, if at all possible, until the intracranial pressure has normalized. The most important modality in the workup of suspected MSCC is gadolinium enhanced MRI of the entire spinal axis. In the initial evaluation of a patient with suspected metastatic spinal cord compression, it is critical to image the entire spine, as 25% of these patients have spinal cord compression verified at multiple levels by MRI, and approximately two-thirds of these have involvement of different regions of the spine (32). In addition, a sensory level present on patient evaluation may be two or more levels different from the actual lesion on MRI in 28% of patients, and four or more levels distant in 21% of patients (32).
General Management
Multimodality therapy for CNS tumors may consist of medical therapy, surgical resection, radiation therapy, or some combination of the above.
Medical Therapy
Medical treatment generally consists of steroids with or without mannitol (33). Patients who present with emergent symptoms are typically treated with dexamethasone. Response to therapy is usually noted within 12 to 18 hours of administration with over 80% of patients showing dramatic improvement by 3 to 4 days after initiation of therapy (34,35). A common regimen in patients receiving radiation therapy is high dose dexamethasone (10–25 mg IV or po) followed by maintenance on oral steroids (4–6 mg three or four times a day), with tapering initiated upon stabilization of symptoms and initiation of therapy, usually over 1 to 2 months (33,36,37). In the setting of MSCC from solid tumors, dexamethasone has been shown to improve rates of surviving with intact gait function (38,39). Side effects of intermediate- to long-term steroid use may include: hyperglycemia, insomnia, emotional lability, thrush, gastric irritation, ulceration and possibly perforation, proximal muscle wasting, weight gain and adiposity (moon facies, buffalo hump, and centripetal obesity), osteoporotic compression fractures, arthralgias with withdrawal, and aseptic necrosis of the hip joints (40). Some of these side effects persist even after steroid withdrawal. Owing to the incidence of steroid-induced complications with dosing longer than 21 days in duration, higher doses and longer tapering schedules should be based on the physician’s assessment of symptom severity and response (39,41,42). Patients should be instructed during tapering to note signs of worsening headaches and/or existing neurological deficits. They should be instructed to resume a higher steroid dose should such symptoms occur and consult their physician. Asymptomatic patients generally do not require corticosteroids and routine use of corticosteroids during radiation therapy in asymptomatic patients should be avoided. Select patients with MSCC may not receive steroids during treatment if they are at high risk of complications due to underlying medical comorbidities, such as peptic ulcer disease, uncontrolled diabetes, or other medical problems that may cause severe or life-threatening problems if exacerbated by steroids (43). Dexamethasone and mannitol drugs decrease peritumoral brain edema by different mechanisms of action and mannitol is therefore often used in steroid refractory patients (44). A common regimen of mannitol is a 20% to 25% solution given intravenously over ∼30 minutes dosed at 0.5 to 2.0 g/kg (45).
Stabilization of the patient in status epilepticus to perform imaging and make management decisions is critical (46). After securing the airway and stabilizing the patient, seizure activity must be terminated as rapidly as possible, especially as failure to control seizures can potentially lead to physical injuries, airway compromise, secondary brain hypoxia/injury, or coma (45,46). Rapid onset/short acting benzodiazepines and phenytoin are commonly used. Recommended initial regimens include 0.1 mg/kg at 2 mg/min of lorazepam or diazepam at 0.2 mg/kg at 5 mg/min. Phenytoin infusion of 15 to 20 mg/kg at <50 mg/min in adults is indicated for seizure activity refractory to benzodiazepines or after truncation of seizures with diazepam (46). There is no clear evidence to support the prophylactic use of anticonvulsants in patients diagnosed with a brain tumor in the absence of documented seizures (47,48).
Surgical Therapy
Surgical resection and/or placement of a shunt are often required for emergent management of brain tumors causing life-threatening hydrocephalus, mass effect, or profound neurologic impairment. This may relieve symptoms enough that other treatment modalities can be initiated. Symptoms are usually related to mass effect, so resection or debulking are often the only logical choices if medical therapy fails to provide improvement in neurologic symptoms. Rapid surgical decompression is the treatment of choice for such problems when surgery can be safely performed based on patient performance status or tumor location. If no neurosurgical team is available, transfer of the patient should be initiated while medical measures are undertaken to stabilize the patient.
Many patients with spinal cord compression are not candidates for laminectomy and are treated with steroids and radiation therapy. Most series in the literature show no difference in outcomes when comparing laminectomy-treated patients to those managed with radiation therapy alone (15,24,49,50). However, a randomized trial evaluating the
benefit of adding surgical decompression to the radiotherapeutic management of symptomatic metastatic spinal cord compression showed that patients who underwent decompressive surgery had a significantly improved median time of gait retention and ability to regain gait function albeit without affecting overall survival (51). Therefore, all patients presenting with MSCC of short duration should be evaluated by an experienced neurosurgeon for emergent decompression before initiating radiation therapy.
benefit of adding surgical decompression to the radiotherapeutic management of symptomatic metastatic spinal cord compression showed that patients who underwent decompressive surgery had a significantly improved median time of gait retention and ability to regain gait function albeit without affecting overall survival (51). Therefore, all patients presenting with MSCC of short duration should be evaluated by an experienced neurosurgeon for emergent decompression before initiating radiation therapy.
Radiation Therapy
Definitive Radiation Therapy
Radiation therapy plays a primary role in the management of most malignant and many benign primary CNS tumors. Table 31.2 provides a referenced overview of the most common primary CNS tumors with International Commission on Radiation and Measurement Units (ICRU) definitions of treatment volumes for initial and (when applicable) boost fields, general dosing guidelines, and outcome endpoints. More detailed descriptions are provided for selected indications later in this chapter.
Palliative Radiation Therapy
Because 60% to 70% of patients who present brain metastases have multiple lesions, radiotherapy is the primary modality for palliation in this setting (52,53). Many patients treated with medical therapy and radiation experience an improvement in their performance status. Radiation therapy dosing schedules for the treatment of emergent patients should take into account their initial response to steroids, the extent of extracranial disease, the primary diagnosis, and its anticipated response to systemic therapy.
Two randomized trials comparing radiotherapy with or without surgical resection in the management of a solitary brain metastasis have documented a survival advantage with the addition of surgery over radiation alone (54,55). However, a third randomized trial was negative (56). There is no level I evidence demonstrating any survival benefit from operating on patients with multiple metastases. However, patients with severe neurologic symptoms from one or more dominant metastases who are unresponsive to medical therapy may benefit from a craniotomy. An improvement in the patient’s performance status can then be followed by external-beam radiation therapy. Acute leukemic brain infiltration is a rare, potentially fatal presentation treated with whole-brain radiation therapy.
Patients with malignant glioma who require emergent treatment are typically treated with surgical debulking and steroid therapy. Patients with a poor performance status who are unable to undergo surgical debulking may be treated with a short course of whole-brain radiation similar to that used for brain metastases.
For metastatic MSCC, there are no randomized trials comparing radiotherapy over best supportive care or medical therapy alone. Every published series of radiotherapy for MSCC has shown that it is effective in relieving pain and maintaining or improving neurologic function. Morbidity is generally low and well tolerated even by patients with a poor performance status. Approximately 89% of patients who are ambulatory before radiation therapy retain gait function, although an average of 39% of patients with paralysis and only 10% of paraplegic patients will remain ambulatory (17).
General Concepts of Radiation Therapy
Workup and Staging
Multimodality Imaging for Simulation, Treatment Planning, and Dose Delivery
The success of modern radiation therapy, besides the introduction of new irradiation technologies, can also partially be attributed to the development and availability of various novel imaging techniques that help to (i) better delineate targets and regions of avoidance, (ii) assess the motion of target and critical structures throughout breathing cycles, (iii) better understand the dosimetrically relevant composition of the tissue in the path of the radiation, (iv) lower the setup uncertainty of the patient, (v) verify the location of the target during dose delivery, and (vi) assess the location of the deposited dose.
A high-resolution CT scan acquired at the time of treatment planning simulation provides a three-dimensional (3D) voxel grid of the patient. In each elementary voxel, the CT software calculates the linear attenuation coefficient of the matter contained in it. Based on this, each voxel gets assigned a shade of gray (for visualization purposes of the tissue) and a CT number also called Hounsfield Unit, which later is translated into physical density in the treatment planning software (for dose calculation purposes). Since the CT modality has poor soft tissue contrast, iodine-based contrast agents are often injected into the blood to better visualize vessels or tumors. Images with sub-millimeter voxel dimensions can be acquired with only a minute or two scanning time.
MRI is a complementary imaging modality to CT. Since it does not involve ionizing radiation and has very good soft tissue contrast, it is one of the most widely used imaging modalities in management of CNS tumors. Its signal is based on relaxation properties of proton spins in a strong external magnetic field. The patient body is again subdivided into voxels, and based on their signal strength received during imaging a shade of gray is assigned to them and the image is reconstructed. Numerous ways (called sequences) have been researched and developed (i) to disturb the spin lattice of patients’ protons in the applied external magnetic field and (ii) to detect their relaxation properties. The two most commonly known techniques are the T1- and T2-weighted sequence, where T1 and T2 are the longitudinal and transverse relaxation times of proton spins, respectively. Generally speaking, the shorter the voxel’s T1, the
more signal it produces and it appears brighter on the scan. On the other hand, the longer the T2, the longer the signal is acquired, making the signal-producing tissue brighter. Water in the bulk phase (e.g., CSF) has long T1 and T2 relaxation times; therefore, it appears dark on T1-weighted but bright on T2-weighted acquisitions. Gadolinium-based contrast agents, which lower T1 relaxation times, are often utilized to enhance brain tumor appearance. The mechanism of their action is that the tumor compromises the brain blood barrier, making the membranes more permeable and letting the contrast agents in. The contrast agent lowers the T1 time making the tumor brighter on T1-weighted images than it was before the contrast administration. Vasogenic edema associated with brain tumors appears bright on T2-weighted images. If fine structures (cranial nerves, inner ear) need to be visualized, that is, extremely high spatial resolution is needed, the constructive interference steady state (CISS, called also FIESTA) sequence is utilized. If hyper-intense abnormalities obscured by CSF within ventricles are needed to be seen, a specialized MRI technique called FLAIR can be utilized to null signal from the CSF.
more signal it produces and it appears brighter on the scan. On the other hand, the longer the T2, the longer the signal is acquired, making the signal-producing tissue brighter. Water in the bulk phase (e.g., CSF) has long T1 and T2 relaxation times; therefore, it appears dark on T1-weighted but bright on T2-weighted acquisitions. Gadolinium-based contrast agents, which lower T1 relaxation times, are often utilized to enhance brain tumor appearance. The mechanism of their action is that the tumor compromises the brain blood barrier, making the membranes more permeable and letting the contrast agents in. The contrast agent lowers the T1 time making the tumor brighter on T1-weighted images than it was before the contrast administration. Vasogenic edema associated with brain tumors appears bright on T2-weighted images. If fine structures (cranial nerves, inner ear) need to be visualized, that is, extremely high spatial resolution is needed, the constructive interference steady state (CISS, called also FIESTA) sequence is utilized. If hyper-intense abnormalities obscured by CSF within ventricles are needed to be seen, a specialized MRI technique called FLAIR can be utilized to null signal from the CSF.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree