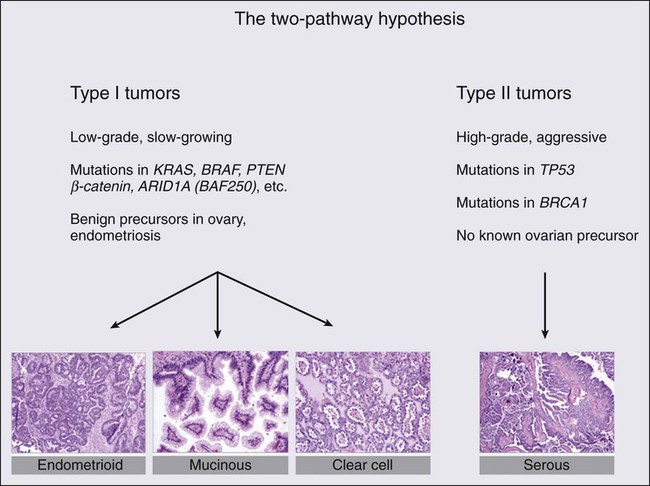
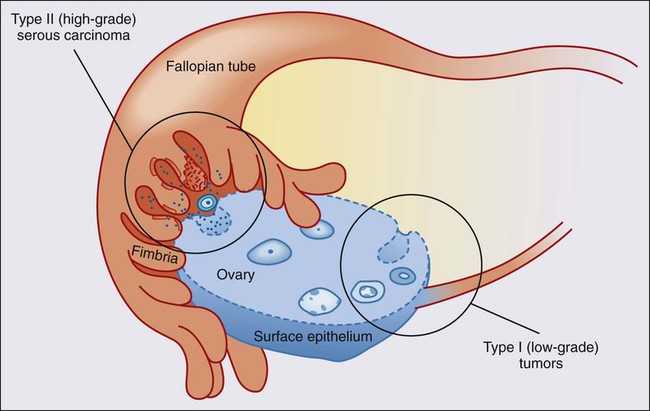
Mark Morgan, Jeff Boyd, Ronny Drapkin and Michael V. Seiden • Ninety percent of ovarian cancers represent a collection of “epithelial tumors” that increase in incidence with age, with patients diagnosed at a median age of 63 years. Stromal tumors represent less than 10% of malignant tumors arising in the ovary and typically present with symptoms related to sex hormone production. The remaining tumors are germ cell tumors that occur in adolescents and young adults. • Lifetime risk of epithelial ovarian cancer is approximately 1 in 85. Risks are lower in multiparous women and those who have taken oral contraceptives. Risks are significantly higher in women with germline mutations in BRCA1 or BRCA2 or other DNA mismatch repair genes. • Epithelial and stromal tumors spread primarily by exfoliation of cells into the peritoneal cavity whereas germ cell tumors tend to spread by lymphatics or hematogenously. • Patients should usually undergo surgical resection of ovarian tumors with comprehensive surgical staging. Most women with epithelial ovarian cancer present with advanced disease and aggressive surgical cytoreduction is recommended. Approximately 10% of these patients will also present with malignant pleural effusions. Comprehensive surgical staging is recommended for stromal and germ cell tumors although the role of lymphadenectomy is unclear when there is no gross lymphadenopathy. • Epithelial tumors are typically treated with carboplatin and paclitaxel as well as maximal surgical cytoreduction. A high percentage of women will experience a complete clinical remission; however, the majority who present with advanced disease will relapse within a few years. Median survivals for women with advanced disease are approaching 5 years. Germ cell tumors typically are treated with bleomycin, etoposide, and cisplatin in regimens initially described in the management of testicular carcinoma with the majority of women cured of disease. • Women with early-stage disease typically receive adjuvant therapy with regimens similar to those used for advanced disease. Management of Recurrent Disease • Most women with epithelial cancer will develop recurrent intraperitoneal tumor. Tumors recurring greater than 6 to 12 months after the prior exposure to platinum are often retreated with platinum-based therapy. Patients with rapid recurrence after platinum therapy may be palliated with a variety of drugs, including liposomal doxorubicin, topotecan, and gemcitabine. In addition, bevacizumab has significant activity in the management of recurrent disease. • Most women with recurrent epithelial cancer will eventually have significant bowel dysfunction. Optimal management often requires a multidisciplinary approach. Ovarian cancer is the leading cause of gynecologic cancer–related deaths and the fifth overall cause of cancer deaths among American women.1 Globally, it afflicts more than 200,000 women each year and claims the lives of over 125,000 annually.2 The high case-to-fatality ratio is in part due to the fact that there is currently no effective screening tool for the early detection of this disease. The vast majority of cases are diagnosed at late stage (International Federation of Gynecology and Obstetrics [FIGO] stage III and IV),3 for which the 5-year survival rate is less than 30%.4 Ovarian cancer is a large collection of tumors that arise or present in a relatively small organ. However, each of these tumor types has very different histologic features and more importantly biological and genetic features that define each of a myriad of malignancies. Tumors are typically divided into epithelial malignancies, stromal tumors, and germ cell tumors. Epithelial malignancy, the most common and lethal of the family of ovarian malignancies, is a misnomer because the ovarian surface epithelium is a mesothelium yet primary ovarian mesotheliomas are extraordinarily rare. Instead, it is likely that epithelial tumors are the result of some still poorly defined and oncogenically induced mesenchymal–epithelial transformation.5 Alternatively, they may represent a metastatic site from the epithelium that initially resided in an extraovarian müllerian site such as the uterus or fallopian tube. Risk factors for ovarian cancer can be divided into those that confer markedly elevated risk compared with those that confer moderate or, more commonly, very modest increased risk of ovarian cancer. In the general population, the lifetime risk of ovarian cancer in a woman is approximately 1.25% or 1 case per 80 female births.1 Globally, ovarian cancer is found at slightly higher incident rates in individuals of western and central European descent with age-adjusted rates of approximately 11 cases/100,000 year. Africans and south central Asians may have rates a few times lower, although it is possible some of these differences are from underreporting.6 Table 89-1 reviews the risk factors associated with ovarian cancer. The highest risk is associated with germline mutations in BRCA1 that confer a lifetime risk of ovarian cancer of 25% to 50%.7–10 Significant elevations in risk are also seen in individuals with germline mutations in BRCA2.10–10 Although less wellstudied, mutation in the mismatch repair genes (MLH1, MSH2, PSM2, MSH6) as seen in hereditary nonpolyposis colorectal cancer (HNPCC) or Lynch syndrome also confer significant risk, which has been estimated in a few studies to be as high as 1% per year for women in their 40s or 50s.11 With the use of more comprehensive yet targeted sequencing of genes associated with DNA repair, it appears there is a heightened risk of ovarian cancer in a variety of individuals with germline mutations in genes such as Check1, Rad51, p53, as well as others.12 Although these germline mutations confer markedly elevated risk, there are several lifestyle or environmental factors associated with moderate or modest impact on risk. Age is a major risk factor, with rates of ovarian cancer rising sharply in perimenopausal years and peaking well past menopause. Other risk factors include increased body mass index, a history of polycystic ovarian syndrome, and perineal talc exposure.13–17 Some risk factors such as endometriosis may confer risk for specific types of ovarian cancer, namely clear cell and endometrioid types.18–19 Table 89-1 Genetic, Lifestyle, and Environmental Factors Associated with Epithelial Ovarian Cancer Risk BMI, Body mass index; HNPCC, hereditary nonpolyposis colorectal cancer; IUD, intrauterine device. There are several protective factors. For example, late menarche, early menopause, early first birth, and breast feeding are associated with a decreased risk of ovarian cancer.14,20–22 Likewise, there is an incremental risk reduction per each year of oral contraceptive use.23 All of these factors are associated with a decrease in the number of ovulatory cycles. However, the reduction in risk may not be explained solely by changes in ovulatory cycles because tubal ligation and intrauterine device (IUD) use are also strongly protective.23,24 Curiously, a history of bone fracture and mumps may be protective and are associated with a higher likelihood of circulating anti-MUC 1 autoantibodies.27–27 Whether this relates to a mechanism of immune surveillance is unclear. Other behaviors may have complex effects, such as cigarette smoking, which increases the risk of mucinous cancer (particularly borderline tumors), decreases the risk of clear cell and endometrioid carcinoma, and leaves the incidence of serous cancer unchanged.28,29 Ovarian cancer is an extraordinarily complex set of malignancies, each characterized by unique histogenesis, early natural history, and molecular genetic features. Although this is evident when epithelial ovarian carcinoma (EOC) is compared with nonepithelial ovarian cancers (e.g., sex cord-stromal tumors), new insights into the etiology and biological characteristics of the more common EOCs are rapidly emerging. Historically, there was wide acceptance of the concept that the epithelial component of the ovary gives rise to four common histologic variants. There was controversy, however, about whether tumorigenesis occurs in the single-cell layer of the surface epithelium or in architectural variations such as cystic inclusions. This concept is problematic from the perspective of embryologic development because EOCs are of serous, endometrioid, mucinous, or clear cell histologies, like their gynecologic counterparts of müllerian origin—the fallopian tubes, uterus, and upper vagina. However, the ovary is not derived from the müllerian duct; it develops separately on the urogenital ridge, the surface epithelium of which is a modified mesothelium, contiguous with, and morphologically resembling, the peritoneal mesothelial lining. Although metaplasia during malignant transformation is a possibility, the question persists as to whether EOC invariably arises from the ovary or perhaps in some cases from müllerian remnants, for example, rete ovarii, lesions such as endometriosis, or other müllerian-derived components of the reproductive tract.30 This section will address various theories and classification schemes for the etiology of EOCs, their fundamental biological features, and the molecular genetic features of EOCs and nonepithelial ovarian cancers. In addition to the well-recognized molecular and biological heterogeneity among EOC histologic variants, a more sophisticated model is emerging for the classification of EOCs based on histology, tumor grade, and early natural history.31 One such model posits that a “type I” tumor category consists of low-grade serous, low-grade endometrioid, clear cell, mucinous, and transitional (Brenner) carcinomas. In the case of low-grade serous tumors and mucinous tumors, they are suggested to arise from corresponding benign cystic epithelium, often through a borderline (low malignant potential) tumor, supporting the classical paradigm of stepwise morphologic progression during tumorigenesis. Type I tumors are generally indolent, of early stage at diagnosis, and genetically stable. Ironically, these tumors are relatively chemoresistant, and thus the prognosis may be poor when metastasis occurs, although survival times are typically long. Clear cell and low-grade endometrioid cancers may be considered a subclass of type I tumors, some proportion of which arise from the malignant transformation of endometriotic lesions that implant on the ovary. In contrast, “type II” tumors are aggressive, generally chemosensitive initially, often presenting at an advanced stage, and include high-grade serous (and other high-grade histologic type) carcinomas, undifferentiated carcinomas, and carcinosarcomas (Fig. 89-1). Type II tumors are considerably more common than type I tumors; the etiology and biological characteristics of high-grade serous tumors, the most common EOC, will be discussed in greater detail. The most remarkable distinction between type I and type II tumors is the absence of mutations in the TP53 gene and low chromosomal instability in the former, and the near ubiquitous presence of TP53 mutations and high chromosomal instability in the latter class of tumors.32,33 In terms of well-characterized candidate genetic alterations in type I tumors, low-grade serous tumors display mutually exclusive KRAS or BRAF mutations, but the proportion of affected cases is now debated.34 Recent data from a whole exome analysis of low-grade serous tumors indicate the presence of very few point mutations; 64 distinct genes were mutated in 8 tumors.35 However, in a validation set, only KRAS and BRAF mutations were reproducibly found. These data support the concepts of relative genetic stability of low-grade serous tumors and that KRAS and BRAF mutations are likely important in their pathogenesis. Other types of genetic alterations, such as translocations and epigenetic alterations, have yet to be adequately explored, however, and clearly, at least 50% of low-grade serous tumors appear not to harbor KRAS or BRAF mutations. Interestingly, KRAS mutations are present in approximately 50% of mucinous carcinomas, of both the gastrointestinal and müllerian subtypes, but BRAF mutations are uniformly absent.36 These molecular characteristics of low-grade serous cancers and mucinous cancers are common in borderline serous and mucinous tumors as well, supporting the hypothesized pathogenic continuum of these two tumor types.37 A common molecular genetic alteration also exists in clear cell and low-grade endometrioid cancers; the ARID1A gene, encoding a key component of the SWI-SNF chromatin remodeling complex, is a tumor suppressor gene mutated in approximately half of clear cell EOCs and in approximately one-third of endometrioid EOCs.38,39 In two patients, the same ARID1A mutations were evident in cancers adjacent to atypical endometriosis, providing strong genetic evidence for a link between endometriosis and these two cases of type I cancers.38 Low-grade endometrioid and clear cell cancers are also characterized by mutations in genes encoding components of the Wnt signaling pathway, including CTTNB1, PTEN, and PIK3CA.40 Finally, the PPP2R1A gene, encoding a subunit of protein phosphatase 2A, is mutated in a small fraction of all four histologic variants of type I EOCs, but mutations are invariably absent in type II tumors.41 An enormous advance in our understanding of the molecular genetic landscape of type II EOCs was facilitated by the Cancer Genome Atlas Project, which analyzed more than 300 high-grade serous EOCs.33 In addition to whole exome, genome, and comprehensive gene expression analyses, gene methylation and copy number alterations were also examined. As noted above, TP53 mutation and a high level of chromosomal instability are present in essentially all high-grade serous tumors. Germline mutation of the BRCA1 or BRCA2 genes was observed in 17% of cases, with somatic mutations in an additional 3% of tumors. Interestingly, mutations in only seven additional genes were observed in as many as 2% to 4% of cases. However, a consideration of other types of genetic aberrations, such as gene amplification, and upregulation or downregulation of gene expression, indicates the presence of oncogenic alterations in the RB pathway in 67% of cancers, alterations in the PI3K/RAS pathway in 45% of cases, and in the NOTCH pathway in 22% of cases. Additionally, defective homologous recombination-mediated DNA repair, as influenced by BRCA1/2 mutation or genetic or epigenetic alterations in additional genes with a role in this process, is present in 51% of cases. Finally, the FOXM1 signaling pathway, affecting cell cycle progression or DNA repair, is altered in 84% of high-grade serous tumors. It is likely that this type of multifaceted approach to the detection of pathogenic alterations in critical pathways, when applied to type I tumors, will yield similar insights. The most enduring theory of ovarian carcinogenesis holds that ovarian carcinomas arise from müllerian metaplasia of the ovarian surface epithelium or subcortical epithelial inclusions and develop as a function of genotoxic stimuli introduced to this epithelium during reproductive years (Fig. 89-2).42 Although this model supports the genesis of type I tumors, very few high-grade serous carcinomas have been encountered at a stage at which their ovarian origin can be determined with confidence and there is scant evidence for the existence of a precursor lesion in the ovarian surface epithelium or circulating immune complexes. Insight into the pathogenesis of high-grade serous carcinomas came from investigating the prevalence of occult ovarian and fallopian tube cancers in women with germline BRCA gene mutations. Inherited mutations in BRCA1 or BRCA2 are associated with familial ovarian and breast cancer syndromes and account for approximately 11% to 15% of ovarian carcinomas.3,4,43,44 Mutations in either gene confer a 15% to 40% lifetime risk of developing ovarian cancer.45 Many women with germline BRCA mutations elect to undergo risk-reducing bilateral salpingo-oophorectomy, after which the ovaries are thoroughly examined for evidence of occult cancer. Until recently, the fallopian tubes were not systematically examined in these cases. Consequently, early-stage tubal cancers have been rarely detected and severely underreported in BRCA mutation carriers. Recent studies suggest that the fallopian tube may harbor a cell-of-origin, the fallopian tube secretory epithelial cell (FTSEC), for high-grade serous carcinomas of the ovary. Supportive evidence for this hypothesis includes (1) ~5% to 10% of BRCA1 mutation carriers undergoing prophylactic surgery will have an early lesion, termed serous tubal intraepithelial carcinoma (STIC) in their fallopian tube fimbria; (2) >50% of women with stage III/IV pelvic serous cancer also harbor a STIC; (3) identical TP53 mutations have been identified in STICs and corresponding serous carcinomas12; (4) nonneoplastic FTSEC and serous carcinoma share similar morphological and immunophenotypic features; and (5) a candidate precursor lesion (the p53 signature) composed of benign-appearing FTSECs that harbor DNA damage and TP53 mutations, has been described in the fallopian tube (Fig. 89-3).45–50 These observations suggest that pelvic serous carcinomas previously assigned to different sites of origin (ovary, fallopian tube, or peritoneum), share a common carcinogenic pathway not previously appreciated, which originates in the FTSEC. This new model of pathogenesis is compelling and also entirely consistent with longstanding epidemiological observations. It has long been documented that lifetime ovulation is positively correlated with high-grade serous carcinoma, and that factors such as parity and birth control, which would decrease lifetime ovulation, have a protective effect.51 During ovulation, the follicular fluid surrounding the ovum is released and bathes the surrounding tissue, including the ovarian surface epithelium and fallopian tube fimbria proximal to the ovary. The composition of this fluid, known to play a critical, albeit poorly understood, role in the development of the follicle, is ill defined but contains hormones, fatty acids, reactive oxygen species, and growth factors that can have mutagenic effects on the surrounding epithelium with each successive ovulatory cycle. In fact, a recent study in a mouse model showed that ovulation causes DNA damage to tubal epithelial cells, suggesting that the distal fallopian tube is susceptible to double-strand DNA breakage during ovulation.52 The shifting model of serous cancer pathogenesis is starting to impact clinical care. Sectioning and extensive examination of the fimbriated end of the fallopian tube is becoming common practice in academic tertiary care centers. Encouragingly, this has resulted in independent validation and support for the fallopian tube model of serous pathogenesis.53–56 The model is also stirring discussion about whether prophylactic surgery should be limited to the fallopian tubes, or whether one of both ovaries may be spared in BRCA mutation carriers. Salpingectomy alone carries an obvious decrease in early menopause–related morbidity and improvement in quality of life, including preservation of fertility, but evidence supporting this approach for the management of high-risk women is still very limited. A related question is whether fimbriectomy is a viable alternative to tubal ligation as a sterilization technique.57 Arguably, sterilization practices that target the fimbria may maximize the protective effect of tubal sterilization on serous cancer prevention. However, the value of such a practice must be evaluated in the context of a clinical trial that carefully evaluates the safety and feasibility of fimbriectomy with long-term follow-up to ensure that it is effective to reducing the risk of “ovarian cancer” in an at-risk population. Nevertheless, barring the emergence of a successful chemopreventive agent or early detection strategy, removing fimbrial tissue is a provocative, albeit yet unproven, surgical approach to reducing serous malignancies. In addition to the aforementioned clinical issues that can now be addressed, this new model has sparked the development of novel fallopian tube–based experimental platforms for the discovery of biomarkers and therapeutic targets.25 Coupled with a recent report from The Cancer Genome Atlas (TCGA), which provided a comprehensive, panoramic view of the genomic complexity of serous carcinomas, we are now poised to leverage these new insights for better patient management and care. The sex cord-stromal tumors account for approximately 7% of all ovarian neoplasms, and consist of two broad categories, granulosa-stromal cell tumors, and Sertoli-stromal cell tumors.58 The majority of these tumors are relatively indolent and are associated with a favorable long-term prognosis. A substantial proportion of sex cord-stromal tumors is diagnosed in patients under 40 years of age; these tumors have the potential to produce steroid hormones. Remarkably, a single somatic missense mutation of the FOXL2 gene is present in the great majority of all adult-type granulosa cell tumors, rare in other granulosa cell tumors, and largely absent in EOCs or other tumor types.59 The FOXL2 gene encodes a transcription factor known to be critical for granulosa cell development, and is likely pathognomonic for adult granulosa cell tumors. Clues to the pathogenesis of Sertoli-stromal cell tumors derive from the observation that germline truncating mutations in DICER1, which encodes an endoribonuclease in the RNase III family essential for processing microRNAs, are observed in families with the pleuropulmonary blastoma-family tumor and dysplasia syndrome, which includes nonepithelial ovarian tumors, including sex cord-stromal tumors.60 Somatic missense mutations of DICER1 are found in about one-third of nonepithelial ovarian tumors, including 60% of Sertoli-Leydig cell tumors. This finding suggests a novel mechanism through which perturbation of microRNA processing may be oncogenic. Finally, women affected by the rare Peutz-Jeghers hamartomatous polyp syndrome, associated with germline mutation of the STK11 (LKB1) serine-threonine kinase gene, are susceptible to sex cord tumors with annular tubules.61 Ovarian germ cell tumors are composed of seven major histologic tumor types derived from the primitive germ cells of the embryonic gonad, and account for approximately 3% of all ovarian neoplasms. Although distinct immunohistochemical features typically distinguish the various histologic types of germ cell tumors, specific genetic alterations have been described only for dysgerminomas, the most common malignant ovarian germ cell neoplasm occurring in pure form. They tend to be tetraploid, with the occasional presence of a small isochromosome, i(12p). Copy number alterations have been identified affecting gains on 12p, 12q, 21q, 22q, and loss on 13q.62 Missense mutation of codon 816 of the KIT oncogene occurs in approximately one-third of dysgerminomas, and is associated with advanced stage at presentation. As such, this mutation represents a potential therapeutic target.63 The existence of a familial breast and ovarian cancer syndrome was formally recognized as early as 1978 by Lynch and colleagues,64 and the BOC syndrome is now a well-accepted clinical entity. The great majority of individuals affected by the BOC syndrome are BRCA1 or BRCA2 mutation carriers. A site-specific manifestation of familial ovarian carcinoma in which an excess of ovarian cancer but not breast or other cancers occurs was also recognized at one time, but genetic linkage analyses failed to demonstrate association of these kindreds to any locus other than the BOC susceptibility gene BRCA1.65 These families are most appropriately considered as affected by a variant manifestation of the BOC syndrome in which breast cancer is rare or undocumented. Significant risks of cancers other than breast and ovarian associated with BRCA1/2 appear to be limited to uveal melanoma, pancreatic, prostate, and male breast cancers in BRCA2 mutation carriers, although the lifetime risks of these cancers are low compared with female breast and ovarian cancers.66 The Society of Gynecologic Oncology developed guidelines to assist in identifying individuals at risk for BOC syndrome,67 and these were also endorsed by the American College of Obstetricians and Gynecologists.68 Following the original reports of genetic linkage of early-onset breast cancer families and some breast and ovarian cancer families to the BRCA1 locus on chromosome 17q,69 the BRCA1 gene was cloned and characterized in 1994.70 Shortly thereafter, the BRCA2 locus on chromosome 13q was defined,71 and the gene was identified in 1995.72 Based on a large volume of literature, a reasonable estimate is that 55% of hereditary ovarian cancers are linked to BRCA1 and 25% to BRCA2 in the context of the BOC syndrome, and 15% to the Lynch syndrome genes. Several additional genes, including RAD51C, RAD51D, and BRIP1, account for up to 5% of hereditary ovarian cancers.73 The BRCA genes share remarkable similarity in both structure and function. Mutations occur throughout both genes, with more than 80% being nonsense or frameshift alterations leading to a truncated protein product; the remainder are missense and other “variants of uncertain significance,” and a very small proportion consist of large-scale gene rearrangements. Well over 1000 distinct inherited mutations in each gene have been described. The prevalence of BRCA mutations in the general population has been estimated to be as high as one in 400. This figure varies considerably among distinct populations, however. Of note, some ethnic or geographically isolated populations carry specific BRCA mutations at a high frequency, generally as a consequence of a founder effect.74 This phenomenon is most pronounced in the Ashkenazi Jewish population, in which approximately 2.5% of individuals carry one of two distinct mutations in BRCA1 (185delAG or 5382insC) or the 6174delT mutation in BRCA2.75 In a search for genotype-phenotype correlations, the study of BOC families revealed the presence of an “ovarian cancer cluster region” in the very large exon 11 gene of BRCA2. Emerging data suggest that a similar region exists in BRCA1, but the mechanism for these effects are not clear. Mutations of the BRCA genes are inherited in an autosomal dominant fashion; somatic loss of heterozygosity at the BRCA loci in ovarian cancers invariably affects the wild-type allele, consistent with their function as classic tumor suppressor genes. Given the very large size and multiple functional domains within the BRCA proteins, it is not surprising that both interact physically with numerous additional proteins and participate in multiple cellular processes. Over the years, a major challenge had been to distinguish among the various functions of BRCA1/2 insofar as they relate directly to tumor suppression.77 Based on a wealth of data from model systems, there is now strong consensus that the primary role of both proteins in this regard is in the repair of DNA double strand breaks through the process of homologous recombination; loss of this function leads to genomic instability and tumorigenesis.80–80 The BRCA1 protein is substantially more diverse with respect to protein–protein interactions, and involvement in protein supercomplexes, playing a role in DNA replication, cell cycle checkpoint control, apoptosis, regulation of transcription, chromatin unfolding, and protein ubiquitination. Although the BRCA2 protein probably also plays a role in cell cycle checkpoint control and regulation of mitosis, its key function appears to involve serving as a scaffold for RAD51 in the repair of DNA double strand breaks, as for BRCA1, through homology-directed DNA repair.81 In carriers of mutant BRCA1 or BRCA2, penetrance is high but incomplete for ovarian cancer. Numerous studies using multiple designs have proposed the lifetime risk of ovarian cancer associated with germline BRCA mutation, with estimates converging around 40% for BRCA1 and 20% for BRCA2. There is considerable evidence that these risks are affected by other genetic and environmental factors clustering in families. Data from large collaborative genomewide association studies suggest that several common alleles modify ovarian cancer risk associated with BRCA mutation carriers.82 The clinical and pathological characteristics of BRCA-linked compared with sporadic ovarian cancer are distinct. The age at diagnosis for BRCA1-linked ovarian cancer patients (age 45 to 50 years) is considerably younger than for those in the general population (age 61 years). In contrast, BRCA2-linked ovarian cancer patients are diagnosed at a significantly older age (55 to 60 years) than BRCA1-linked patients.83,84 Notably, in contrast with hereditary breast cancer, BRCA-linked ovarian cancers do not occur in women under 30 years. These data have considerable implications for clinical intervention in unaffected mutation carriers. The histopathological characteristics of BRCA-associated ovarian cancers differ from the spectrum associated with their sporadic counterparts. In the BRCA population, tumors of high-grade serous histology are overrepresented, although endometrioid and clear cell tumors are also observed. Tumors of mucinous histology and borderline (low malignant potential) tumors do not occur in association with BRCA mutation. In addition, low-grade serous ovarian cancers are the result of a distinct pathogenic process, and do not occur in the BRCA population. Finally, there does not appear to be a difference between the histopathological spectrum of tumors in the BRCA1 compared with the BRCA2 population.85 The clinical course of BRCA-linked ovarian cancer patients is dramatically different from that of sporadic ovarian cancer patients matched for all other clinical and pathological criteria of prognostic significance. Specifically, progression-free survival and overall survival are significantly improved in BRCA mutation carriers. This prognostic difference has been unequivocally demonstrated in both the Ashkenazi Jewish founder population83,86 as well as the general population,87,88 when comparing BRCA-linked tumors with sporadic tumors. Furthermore, the improved outcome appears to be greater for BRCA2 mutation carriers compared with BRCA1 mutation carriers. The biological basis for this phenomenon is also reasonably well established based on work in vitro and in model systems in vivo. Platinum-based combination chemotherapy, the standard first-line treatment for epithelial ovarian carcinoma, is an “accidental” targeted or personalized therapy in this context. Cis(carbo)platin is known to create interstrand DNA cross links, which are repaired by the cell through creation of a double-strand break at the crosslink, which is then repaired through homologous recombination. Cells deficient in homologous recombination-mediated DNA repair are thus hypersensitive to the cytotoxic effects of platinum-based therapy (as well as other agents that act through similar mechanisms, e.g., mitomycin C).89 The screening of individuals from the general population for hereditary predisposition to the BOC syndrome is a complex and controversial topic. Several groups have made consensus recommendations on this topic, all referencing different criteria, including the American College of Medical Genetics, the National Comprehensive Cancer Network, the American Society of Clinical Oncology, and the American College of Obstetricians and Gynecologists. In 2005, the U.S. Preventive Services Task Force published its recommendation statement. To summarize, the recommendation is against routine referral for genetic counseling or BRCA genetic testing for women whose family history is not associated with an increased risk for a deleterious BRCA mutation (grade D recommendation), whereas the recommendation for women whose family history is associated with an increased risk for deleterious BRCA mutation be referred for genetic counseling and evaluation for BRCA testing (grade B recommendation).90 Although it is generally accepted that women with germline BRCA mutations should be routinely screened for ovarian cancers, there are no data to suggest that ovarian cancer screening leads to improved survival in the high-risk population. An evaluation of 13 ovarian cancer screening studies of high-risk women found that of 70 screen-detected tumors, only 24% were early-stage, similar to the incidence in the general population. This finding suggests that there is likely to be little difference in survival between screened and unscreened populations.91 In the context of prevention, essentially all published retrospective and prospective studies of the effect of risk-reducing bilateral salpingo-oophorectomy (RRSO) in BRCA mutation carriers demonstrate a significant reduction in the risk of ovarian cancer and, generally, breast cancer as well. Typical is the largest and most recent prospective, multicenter cohort study of 2482 women with BRCA mutations.92 In women undergoing RRSO, the procedure was associated with a substantially lower risk of ovarian cancer, first diagnosis of breast cancer, all-cause mortality, breast cancer–specific mortality, and ovarian cancer–specific mortality. These and a wealth of similar data from the literature contribute to consensus recommendations from several professional organizations and experts in the field, including a review of the literature by the Society of Gynecologic Oncology Clinical Practice Committee, which recommends RRSO in the BRCA population.93 The frequent finding of occult ovarian and tubal carcinomas following RRSO reinforce this recommendation, as well as the importance of careful pathological examination of the entire specimen following surgery (see previous section on Etiology and Biological Characteristics—High-Grade Serous Cancer). Hysterectomy is not recommended in this context because there is no evidence for an increased risk of uterine cancer in the BRCA population; however, the use of tamoxifen or estrogen replacement therapy in some patients may affect this decision. Factors such as current age, child-bearing considerations, and BRCA1 versus BRCA2 mutation status contribute to the timing of the procedure with respect to patient age at the time of RRSO, but the consensus recommendation is to perform the procedure no later than age 40 years. Cost- and comparative-effectiveness studies clearly support this recommendation in the context of cancer prevention in BRCA mutation carriers.94,95 Also well established is the protective effect of oral contraceptives on ovarian cancer risk in the BRCA population. In a large representative study, duration of oral contraceptive use increasingly reduced the risk of ovarian cancer by 50% to 60% with up to 5 years of use.96 Historically, use of this chemoprevention strategy has been tempered by conflicting data on an association with increased risk of breast cancer in BRCA mutation carriers. However, a recent meta-analysis of available data concludes that there is no evidence that recent oral contraceptive formulations increase breast cancer risk in BRCA carriers.97 Nevertheless, as with most medical interventions, a discussion between women and their physicians of the benefit–risk ratio is strongly indicated. A history of the recognition and clinical definition of this syndrome is instructive with respect to implications for risk assessment, screening, and prevention. In 1913, Alfred Warthin described “cancer family G,” observed to have an excess of gastric and uterine cancers, but after several generations of observation, members were also documented to suffer from excessive colorectal and extraintestinal tumors.98 Subsequent studies of similar families by Henry Lynch and colleagues lead to a description of the “cancer family syndrome.”99,100 In 1984, the terms Lynch syndrome I and Lynch syndrome II were proposed by Boland and Troncale as corresponding to site-specific familial colorectal cancer and the cancer family syndrome, respectively, without antecedent polyposis.101 Recognizing that Lynch syndromes I and II are manifestations of the same polygenic-based cancer predisposition syndrome, corresponding to the cloning and characterization of the genes responsible for this syndrome in the early 1990s (see later), several collaborative groups, the first meeting in Amsterdam, began proposing criteria designed to provide a uniform basis for diagnosis of a single clinical entity that came to be known as hereditary nonpolyposis colorectal cancer (HNPCC) syndrome.102 The so-called Amsterdam criteria (and later the revised Amsterdam II criteria)103 are very specific for identifying likely gene mutation carriers but are relatively insensitive. They are therefore criticized for being overly exclusive when used as a guide for referring individuals for genetic consultation. Recognizing that there was little consensus with respect to the criteria or threshold for recommending genetic testing for HNPCC, the National Cancer Institute Workshop on HNPCC Syndrome created a set of criteria that, when met, warrant genetic screening.104 These criteria have become known as the Bethesda Guidelines, which appear to be substantially more sensitive but less specific than the Amsterdam criteria as identifying HNPCC kindreds with pathogenic mutations (Box 89-1). This test, described below, is relatively simple and inexpensive, and a negative result translates into a very low probability of the existence of a pathogenic mutation predisposing to what, coming full circle, is now widely referred to simply as Lynch syndrome. Clues to the genetic basis of Lynch syndrome first emerged in 1993, with several independent observations of somatic hypermutability of a class of DNA repetitive elements, known as microsatellites, in sporadic and familial colorectal tumors.105 This observation was accompanied by reports of the genetic linkage of Lynch syndrome kindreds to two loci, one on chromosome 2p and another on chromosome 3p. Identification and cloning of the relevant genes at these loci, MSH2 on chromosome 2106 and MLH1 on chromosome 3107 quickly followed, together with the realization that these genes encoded human orthologs of yeast DNA mismatch repair proteins. The microsatellite instability phenotype previously observed in colorectal and other cancer types associated with the Lynch syndrome could be explained by loss of function of these DNA mismatch repair genes. In Lynch syndrome, one of these genes is inherited in a mutant form through the germline, whereas in sporadic colorectal, endometrial, and gastric carcinomas affected by microsatellite instability, somatic silencing of MLH1 through promoter hypermethylation is the primary pathogenic mechanism.108 Most patients (90%) with Lynch syndrome carry a mutation in either MSH2 or MLH1, with approximately 10% of cases attributable to MSH6 mutation and a very small number to PMS2 mutation. The DNA mismatch repair genes responsible for Lynch syndrome are classical tumor suppressor genes that sustain loss of function mutations; they are inherited in an autosomal dominant fashion with somatic loss of the wild-type allele required for tumorigenesis. The basis for incomplete and variable tissue-specific penetrance of these genes remains unknown. In women, Lynch syndrome is most frequently associated with colorectal and endometrial cancers, with a 40% to 60% lifetime risk of each.109 Other cancers, especially of the gastrointestinal and genitourinary tracts, are common.110 The lifetime risk estimates for EOC associated with Lynch syndrome range from 6% to 20%, and the clinicopathological features of these cancers appear to be distinct compared to ovarian cancer in the general population. Although data are limited, in the two largest studies to date, the mean age at diagnosis is mid-40s, most cancers are well or moderately differentiated endometrioid, clear cell histologic types are overrepresented, and early-stage cancers are relatively common.111,112 Additionally, synchronous endometrial cancers are observed in approximately 20% of cases. There is some evidence that MSH6 may account for a higher proportion of gynecologic, including ovarian cancers. The prevalence of DNA mismatch repair gene mutations in the general population is estimated at 1 in 500 to 1 in 1000,85 and the proportion of all ovarian cancers arising in the context of Lynch syndrome is estimated at 1% to 4%.113 With respect to screening tumor tissues for the possible presence of Lynch syndrome, it is necessary to incorporate the risk of endometrial cancer into guidelines for females, and numerous consensus recommendations are published. The Bethesda Guidelines are sensitive but have a low specificity, and are now less commonly used in the clinical setting. Another approach uses algorithms that incorporate the types of cancer in a family, the age of occurrence, and the relationships of family members with cancer to estimate the likelihood of an individual having Lynch syndrome; several of these computerized algorithms are available online.114 Recently, cost-effectiveness and other considerations have led to an emerging consensus that reflex testing of all incident colorectal, and perhaps endometrial, cancer cases, using immunohistochemistry to assess mismatch repair gene expression, is the most appropriate initial screen for Lynch syndrome.116–116 Of note, 92% of ovarian cancers associated with germline mismatch repair gene mutation demonstrate loss of gene expression in the corresponding gene as assessed by immunohistochemistry.112 Although consensus recommendations uniformly endorse increased surveillance for colorectal cancer in this population, based on the resultant decreased mortality, evidence is lacking for an effective methodology for screening women for gynecologic malignancies. There is, however, evidence for the efficacy of prophylactic, or risk-reducing, surgery. In a retrospective cohort of 315 women with mismatch repair gene mutations, in which 61 had prophylactic hysterectomy with or without bilateral salpingo-oophorectomy, matched to women without surgery and followed for approximately 10 years, no ovarian cancers developed in those who had surgery, whereas 12 (5.5%) who did not developed ovarian cancer.117 Using a different approach, data from a decision analytic model that considered 10,000 theoretical women with a mismatch repair gene mutation indicated that only 28 prophylactic surgeries would be needed to prevent one ovarian cancer in this population.118 Risk-reducing surgery, compared with surveillance, is also the most cost-effective option from a societal healthcare cost perspective.119 Taken together, available data would suggest that prophylactic hysterectomy and bilateral salpingo-oophorectomy be offered to all women genetically susceptible to Lynch syndrome at the age of 35 years, or once child-bearing is complete, following a careful discussion of the risks, benefits, and limitations of this procedure. Approximately 70% of women with epithelial ovarian cancer are seen with advanced-stage disease where lethality is high, with approximately 90% of these women eventually experiencing a recurrence of their disease and most eventually succumbing to platinum-resistant disease. In contrast, the 30% of women who present with early-stage disease enjoy significantly better short- and long-term outcomes, with some reports suggesting 60% to 70% of these women are cured.120 Thus, it would seem intuitive that an effective screening test that detects disease confined to the ovary should produce a higher portion of patients cured of their disease and a corresponding decrease in ovarian cancer–associated mortality. In addition, soon after the discovery of CA-125 in the 1980s, it was appreciated that the majority of women with advanced-stage disease had elevations of this biomarker and subsequent studies identified that 40% to 70% of women with early-stage disease had an elevation of this marker as well.120–125 Thus for the last 15 years there has been a concerted effort to determine whether pelvic ultrasound, CA-125, or the combination might spare women from presenting with advanced-stage disease. In subsequent years, the discovery of additional biomarkers such as human epididymis-4 (HE-4) as well as panels of biomarkers or even more complex proteomic profiles have at first glance offered the hope of early detection. Despite these challenging statistics, a clinical trial reported in the 1990s suggested that large-scale screening trials might be feasible and CA-125 might meet that requirement. In particular, Jacobs and colleagues reported a randomized trial involving over 20,000 women with half randomly assigned to annual blood draws on 3 successive years for CA-125 determination and the remaining half randomly assigned to standard care.126 In the screened group, approximately 4% of the women had a CA-125 of greater than 30, triggering a pelvic ultrasound. Twenty-nine women ultimately underwent surgical exploration to detect six ovarian cancers (positive predictive value of 20%). Over the next 8 years, 10 additional women were found to have ovarian cancer between screens. Ovarian cancer developed in 19 women in the unscreened group, with more high-grade tumors in this unscreened group. Although there were 9 and 18 deaths in the screened versus unscreened populations, this did not reach statistical significance.126 In addition, two large follow-up studies have been conducted. In the United States, the Prostate Lung-Colon-Ovary (PLCO) screening trial invited 78,000 women aged 55 to 74 years to participate. Women were randomly assigned one to one, with 39,000 women assigned to six annual CA-125 serum determinations and four annual transvaginal ultrasound examinations. The remaining 39,000 women were not screened. As might be expected, the screened group had more ovary cancers detected (212) compared with the unscreened group (176).127 Stage and grade of tumors at diagnosis were similar in both groups. Screening generated a significant consumption of resources. Although 212 women in the screened group had a diagnosis of cancer, there were 3285 false positives, with 1080 surgical procedures, and 163 significant complications resulting from screen-associated procedures. Most sobering was the fact that 118 women died of ovarian cancer in the screened group as compared with 100 women in the unscreened group.127 Subset analysis of a portion of these serum specimens using a larger collection of serum biomarkers failed to demonstrate that any panel of biomarkers outperformed CA-125 in early detection. It is possible that HE-4 or some secondary collection of biomarkers might prove to be a superior second-stage screening test in individuals with an elevated CA-125 as compared with transvaginal ultrasound. A second study, based in the United Kingdom under the acronym of the UKCTOCS trial, compared an ultrasound-screened population (n = 51,000) with a CA-125-screened population (n = 51,000) and an unscreened control population (n = 100,000). Unlike, earlier trials, the group of women assigned to CA-125 screening were triaged not only on their CA-125 value but also the rate of change in CA-125 as observed over time using a Risk of Ovarian Cancer Algorithm (ROCA). Individuals whose ROCA analysis crossed a specific threshold were referred for ultrasound in a second round of screening (this arm was referred to as the multimodality screening arm). Although the final survival data will not be available for several years, the results of the prevalence screen have been reported and demonstrated that ultrasound alone was associated with an unacceptable false-positive rate. The multimodal screening rate demonstrated sensitivity, specificity, and positive predictive values of 89.4%, 99.8%, and 43.3%, respectively.128 Ovarian cancer death rates, arguably the only meaningful value, will be available in a few years. Until that time, none of the major medical societies recommend screening in average-risk asymptomatic women. Early-stage ovarian cancer is usually found during the evaluation of a pelvic mass detected either by pelvic examination or radiologic imaging (most commonly ultrasound). Occasionally, it is found incidentally after radiologic evaluation for other medical indications. Although ovarian cancer has been thought of as a “silent disease,” presenting with minimal symptoms until advanced stages, recent evidence suggests that clinical symptoms frequently predate the diagnosis by 3 to 6 months. A “symptom index” developed by Goff based on abdominal bloating, increased abdominal size, difficulty eating, and early satiety identified 57% of women with early disease and 80% of women with advanced disease in a case-control study.129 Although these symptoms are common in women presenting to primary care clinics, the frequency, duration, and severity were worse in the women with ovarian cancer. Other studies have not confirmed the usefulness of the symptom index,130 but studies in this area are ongoing and hope to at least raise the awareness of women and primary care providers to potential symptoms that should be evaluated further.131 If a mass suspicious for ovarian cancer is detected on physical examination or ultrasound, then further imaging such as computed tomography (CT) may be indicated. A serum CA-125 may also be helpful, although it is less specific in premenopausal women where conditions such as endometriosis, pelvic inflammatory disease, or leiomyomas may cause it to be elevated. The CA-125 can also be elevated in pregnancy. Although elevated in about 80% of women with epithelial ovarian cancer, it is also normal in approximately 50% of women with early ovarian cancer.132 Recently, other tests have been evaluated to increase the sensitivity and decrease the false positives associated with the CA-125, especially in premenopausal women. None of these tests to date increase the positive predictive value enough to be used in screening, but they may be useful in prompting referral to surgeons experienced in the management of gynecologic malignancies when a suspicious mass is detected. Several studies have shown that evaluation and treatment outcome is superior for women treated primarily by gynecologic oncologists. However, in the United States, only about one-third of patients are referred to gynecologic oncologists for their primary surgery.133 Currently, a multiple marker assay that includes CA-125-II is available and FDA approved (OVA-1) for use in women with a pelvic mass for whom surgery is planned. This test has a high negative predictive value (96%), allowing a clinician to feel confident that if normal, the mass is unlikely to be cancer and referral to a specialist may not be required. However, it should not be used as a screening test or to determine if surgery should be performed.134 Another blood test, an immunoassay for the HE-4 protein, has been approved for monitoring for recurrence or progression of ovarian cancer. This test is also not sensitive or specific enough alone to be used as a screening test but may prove useful in eliminating some of the false positives associated with the CA-125 (such as endometriosis or pelvic inflammation) when evaluating a pelvic mass.135,136 An initial surgical approach is important for diagnosis and staging of presumed early disease and cytoreduction or debulking of advanced disease. The FIGO staging system for ovarian cancer is most commonly used and is based on the findings at surgery (Table 89-2). Traditionally, a large vertical midline incision has been recommended for both staging and debulking. However, this recommendation is being challenged by some who argue that advances in preoperative imaging and minimally invasive surgical capabilities can make alternative surgical approaches, such as laparoscopic or robotic-assisted surgery, viable alternatives in selected cases.139–139 This is especially true with the more frequent use of neoadjuvant chemotherapy when large bulky tumors may be effectively cytoreduced by treatment prior to surgery. Table 89-2 International Federation of Gynecology and Obstetrics Staging System for Ovarian Carcinoma Data from the New FIGO stage grouping.7 Ovarian cancer spreads primarily by exfoliation into the peritoneal cavity, direct spread, or lymphatic dissemination. Subclinical metastatic disease can be found in approximately 30% of women with an apparent localized cancer, with retroperitoneal lymph nodes being involved about 10% of the time.140 Therefore, a systematic approach to surgical staging is recommended. This involves obtaining washings for peritoneal cytology, omentectomy, careful inspection of the entire peritoneal cavity, random biopsies of pelvic and abdominal peritoneal surfaces, bilateral pelvic and paraaortic lymph node sampling, and in most cases a total hysterectomy and removal of both fallopian tubes and ovaries. Frozen section should be obtained and for young women desiring childbearing, the uterus and contralateral ovary can almost always be preserved when a germ cell or sex cord-stromal tumor is found because they are rarely bilateral. This is also true for low malignant potential (borderline) epithelial tumors and low-grade serous tumors on frozen section. Invasive cancer is found in 20% to 30% of these cases on final pathology. Therefore, complete staging is still indicated for these tumors. In the absence of enlarged lymph nodes, lymphadenectomy may be omitted in sex cord-stromal tumors and possibly even germ cell tumors.141 Although lymph node metastasis are more common in germ cell tumors (especially dysgerminoma), adjuvant treatment decisions can usually be made based on histology. More than two-thirds of patients with epithelial ovarian cancer will present with disseminated disease (stage III or greater). In these cases, women almost always present with weeks or months of increasing gastrointestinal or urologic symptoms, often prompting evaluation from multiple specialists including gastroenterologists, urologists, gynecologists, and sometimes even psychiatrists to consider such diagnoses as irritable bowel syndrome, gastritis, interstitial cystitis, menopause, or depression. In these patients, preoperative evaluation typically demonstrates a complex adnexal mass with cystic and solid components as well as ascites, peritoneal deposits, and a pleural effusion (particularly on the right side). CA-125 is typically elevated in women with serous carcinoma, the most common histologic subtype. As described above, surgical exploration to confirm the diagnosis and proceed with an attempt at maximal surgical cytoreduction is frequently the primary step in the management of these patients. Griffiths in 1975 demonstrated that the amount of residual disease after surgery was a significant prognostic factor in advanced ovarian cancer, and this observation has been confirmed in multiple subsequent studies.142,143 Retrospective review of large GOG randomized trials and the Scottish Randomized Trial in Ovarian Cancer (SCOTROC-1) has suggested that a large part of the benefit in their trials was due to the inclusion of subsets of patients who either started with minimal extrapelvic disease or who did not require extensive surgery to obtain “optimal” status.144,145 Optimal debulking surgery has generally been defined either as no residual tumor nodule >2 cm in diameter, or more recently, no tumor nodule >1 cm in diameter. More recent data suggest that even patients with extensive initial metastatic disease requiring radical surgery of the upper abdomen and diaphragm can obtain improved outcomes only if all gross disease is resected.146,147 This is understandable because (1) there is significant interobserver difference in clinical measurement and (2) the designation of ≤1 or ≤2 cm generally implies diffuse small volume residual disease or carcinomatosis. It would be unusual to be considered “optimally debulked” if there were only a few small nodules remaining. If that were the case, these few nodules would usually be resected. A meta-analysis of 6885 patients with stage III and IV ovarian cancer by Bristow in 2002 demonstrated that in any given cohort of patients, each 10% increase in the proportion of patients undergoing maximal cytoreduction was associated with a 5.5% increase in median cohort survival time.148 Further, Aletti147 found that surgeons at the Mayo Clinic who were more willing to perform radical debulking procedures had improved survivals compared with surgeons less likely to do those procedures for the same disease burden. In reviewing this study and the available data of five other single-institution series and eight clinical trials published since 2003, Chang and Bristow concluded that (1) complete cytoreduction to no gross residual disease is associated with significantly longer overall survival, (2) radical cytoreductive surgery is able to at least partly counteract the effect of tumor burden, and (3) the survival outcome of patients with advanced ovarian cancer is strongly influenced by the individual surgeons ability and willingness to undertake radical surgical procedures to achieve minimal residual disease.149 Three randomized trials have been completed addressing the issue of debulking surgery in the primary management of epithelial ovarian cancer. Two trials, one European and the other American, evaluated patients who had a very unsuccessful primary debulking procedure and explored the value of a second surgical procedure after several cycles of chemotherapy. This strategy of interval debulking surgery was based on the hypothesis that chemotherapy would chemically debulk patients, making surgical cytoreduction possible. Both trials randomly assigned patients to either six cycles of chemotherapy or “interval debulking surgery” after three cycles of cisplatin-based chemotherapy. Both trials gave an additional three cycles of chemotherapy to the patients randomly assigned to interval debulking surgery. The European trial, conducted by the European Organization for Research and Treatment of Cancer (EORTC) and using cisplatin and cyclophosphamide, showed a statistically significant 6-month improvement in median survival for patients undergoing interim cytoreduction.150 The American Gynecologic Oncology Group (GOG) trial, using cisplatin and paclitaxel, showed no significant difference in progression-free and median survival (Table 89-3).151 It is possible that the use of paclitaxel in the GOG trial eliminated the benefit of interval debulking surgery. Also, the initial surgery in the GOG trial was more often performed by gynecologic oncologists than by general gynecologists as in the European trial. This approach resulted in patients starting the GOG trial with less disease and therefore on average they did not receive as large a benefit in the subsequent debulking procedure. In the GOG trial, only 36% of patients were converted from suboptimal to optimal versus 45% of patients in the EORTC trial. Both arms of the GOG trial had a superior median survival than the interval debulking arm of the EORTC trial. Table 89-3 Results of Two Studies of Interval Surgical Cytoreduction
Cancers Arising in the Ovary
Introduction
Epidemiology
Risk Factor
Magnitude of Risk
BRCA1 mutation
↑↑↑
BRCA2 mutation
↑↑
HNPCC
↑↑
Endometriosis
↑
Nulliparity
↑
High BMI
↑
Estrogen around menopause
↑
Early menarche and/or late menopause
↑
Perineal exposure to talc
↑
Protective Factors
Magnitude of Protection
Oral contraceptives
↓↓
IUD
↓
Tubal ligation
↓
Multiple births
↓
Late menarche and/or early menopause
↓
History of mumps
↓
History of bone fracture
↓
Biological and Molecular Genetic Characteristics
Molecular Genetics of Type I Tumors
Molecular Genetics of Type II Tumors
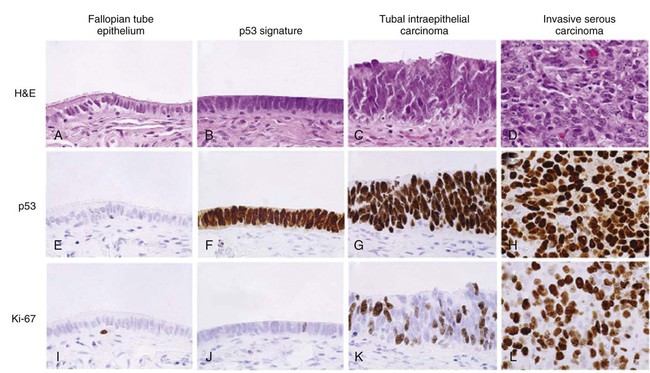
Pathogenesis of High-Grade Serous Carcinoma
Sex Cord-Stromal Tumors
Germ Cell Tumors
Breast and Ovarian Cancer Syndrome
Genetics of the BOC Syndrome
Ovarian Cancer Associated with the BOC Syndrome
Screening, Surveillance, and Risk Reduction in the High-Risk Population
Lynch Syndrome
Genetics of Lynch Syndrome
Screening, Surveillance, and Risk Reduction in the Lynch Syndrome Population
Screening in the General Population
Clinical Manifestations, Patient Evaluation, Staging, and Debulking
Stage
Description
I
Growth limited to the ovaries
IA
One ovary; no ascites; capsule intact; no tumor on external surface
IB
Two ovaries; no ascites; capsule intact; no tumor on external surface
IC
One or both ovaries with either surface tumor; ruptured capsule; or ascites or peritoneal washings with malignant cells
II
Pelvic extension
IIA
Involvement of uterus and/or tubes
IIB
Involvement of other pelvic tissues
IIC
Stage IIA or IIB with factors as in stage IC
III
Peritoneal implants outside pelvis and/or positive retroperitoneal or inguinal nodes
IIIA
Grossly limited to true pelvis; negative nodes; microscopic seeding of abdominal peritoneum
IIIB
Implants of abdominal peritoneum ≤2 cm; nodes negative
IIIC
Abdominal implants >2 cm and/or positive retroperitoneal or inguinal nodes
IV
Distant metastases
Parameter
All
IDS
No IDS
EORTC STUDY55
Patients
408
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access