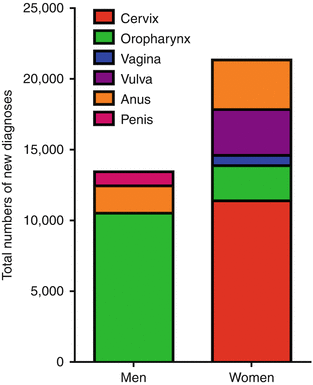
Exposure to HPV occurs with sexual debut and is essentially endemic [4–7]. Infections are asymptomatic, and although most are transient, resolution can take 1–2 years [8–10]. Older women take longer to clear infections, as do smokers, and women with underlying immunosuppression [8]. The clinically silent nature of these infections facilitates maintenance of a large herd burden of transmissible HPV. Moreover, rates of preventive vaccination in eligible US cohorts, young people aged 9–26, have been suboptimal. In 2015, 43% of eligible girls and 28% of eligible boys had completed all three vaccinations in the three-vaccination regimen [11]. Existing prophylactic vaccines, Gardasil© and Cervarix©, are approved for prevention of cervical, vulvar, vaginal, and anal cancers. Because HPV is the underlying cause of a subset of oropharyngeal squamous cell carcinomas (OSCC), which are restricted to the epithelium of the palatine tonsil and base of the tongue in mostly young, nonsmoking, and non-heavy drinking men, their usage as prevention for oropharyngeal cancers is currently being investigated [12, 13].
15.1.1 Biology of HPV Infection
HPVs are non-enveloped, double-stranded DNA viruses which are tropic for mucosal tissues. The genome consists of three functionally divided regions: (a) a noncoding regulatory region; (b) an early proteins region, which encodes for six early proteins (E1, E2, E4–E7); and (c) a late region which encodes for the viral capsid proteins L1 and L2 [14, 15]. HPVs infect basal epithelial (skin or mucosal) cells. Over 200 HPV genotypes have been identified. Oncogenic genotypes include HPVs 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 66 [16].
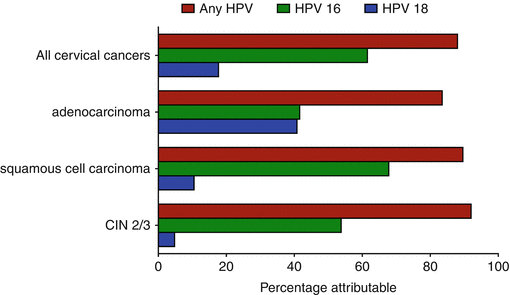
The development of squamous cervical cancer, other anogenital cancers, and head and neck cancers occurs in the setting of a persistent infection with an oncogenic HPV. Virtually all squamous cell carcinoma of the cervix (SCCx) and its precursor, high-grade squamous intraepithelial lesions/cervical intraepithelial neoplasia 2/3 (HGSIL/CIN2/CIN3), are caused by HPV, most commonly, HPV 16 (Fig. 15.3) [17]. Most cervical cancers are attributable to HPVs 16 and 18.
The development of both cervical cancer and HGSIL/CIN2/CIN3 is associated with integration of the HPV genome into the host genome and subsequent expression of two HPV early gene products, E6 and E7, which inactivate p53 and pRb, respectively [18, 19]. Viral integration sites, although randomly distributed within the human genome [20], occur principally at sites where human DNA is prone to breakage (e.g., fragile sites) and appear to affect only the expression of the HPV genome itself. Specifically, E1 and E2 are most frequently disrupted in integration, while the E6 and E7 viral oncogenes are retained, resulting in constitutive expression. Expression of both E6 and E7 is functionally required to initiate and maintain neoplastic transformation [21, 22]. Morphologically, at the cellular level, high-grade intraepithelial lesions are characterized by a high nuclear to cytoplasmic ratio. Histologically, high-grade lesions display full thickness, lack cell maturation, and are mitotically active (Fig. 15.4).
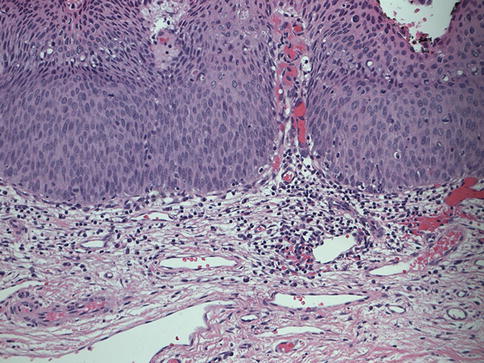

Fig. 15.4
Hematoxylin and eosin staining of CIN 2/CIN3 tissue taken with 20× magnification
15.1.2 Systemic Immune Responses in Natural Infection
In the setting of a natural HPV infection, both humoral and adaptive systemic endogenous immune responses to HPV antigens required for disease initiation and persistence are weak. After viral clearance, antibody titers are detectable in 50–70% of persons [23, 24]. Most memory B cells elicited by natural infection generate antibodies that have low avidity, are nonneutralizing, and do not necessarily protect against reinfection with the same HPV genotype [25, 26]. Similarly, T cell responses to viral antigens are marginal, require ex vivo stimulation to be detectable, and do not reliably distinguish persons whose lesions will regress from those whose lesions will not [27–30]. In point of fact, persistent HPV infections and preinvasive HPV lesions are limited to the squamous mucosa and are presented in a noninflammatory context without systemic viremia. Infections are anatomically restricted to the mucosal epithelium and do not elicit systemic symptoms [5, 8].
Using ex vivo stimulation, HPV 16 E7-specific cytotoxic T cells have been detected in the peripheral blood of persons who have HPV 16+ CIN2/CIN3 [27, 29] and cervical cancer [31, 32]. However, to date, no clear association has been made between the magnitude of response to E7 in the blood and clinical outcomes. In contrast, peripheral blood cytotoxic T cell responses to HPV 16 E6 have been linked to clinical outcomes. In particular, a CD4+ T cell response to E6 is has been associated with better clinical outcomes [33]. Conversely, functionally impaired E6-specific CD4+ T cell responses have been associated with cervical cancers [34].
Despite the rarity of HPV-specific memory T cells in the blood, a subset of persons with CIN2/CIN3 do mount an effective response; not all CIN2/CIN3 lesions progress to invasive cancer. We and others have reported that in a timeframe of 4–6 months, ~35% of CIN2/CIN3 lesions undergo spontaneous regression [35–37]. Similarly, in a Phase II clinical trial testing VGX-3100, a therapeutic synthetic DNA vaccine targeting HPV 16/18 E6 and E7, in persons with HPV 16 or 18+ CIN2/CIN3 prior to planned standard therapeutic resection, 30% of subjects who received placebo experienced histopathological regression [38]. Lesions resulting from mono-infection with HPV 16 were less likely to undergo regression than lesions caused by other HPV genotypes: in this timeframe, ~20–25% of HPV 16-associated CIN2/CIN3 lesions regressed [38].
15.1.3 Tissue-Localized Immune Responses to Natural Infection
The fact that neither the magnitude nor the breadth of naturally occurring T cell responses detected in the blood is robust predictors of regression of preinvasive HPV disease of the cervix raises the question of whether it is possible to identify factors in the target lesion that could predict disease outcome or characteristics of the immune response that eliminate either infection, incipient malignancy, or cancer. Tissue-based studies of HPV lesions are identifying factors associated with clinical outcomes. In CIN2/CIN3 lesions that do not regress, although CD8+ T cell infiltrates in the mucosa are robust, they are largely restricted to the stroma, failing to access the lesional epithelium [39]. The presence of intraepithelial CD8+ T cells, on the other hand, is associated with subsequent regression. Similarly, in HPV-associated cancers of the cervix and oropharynx, the presence of intra-tumoral CD8+ T cells is associated with better prognosis [31, 40, 41]. The fact that despite the presence of tumoral T cells, a persistent HPV infection can lead to progression to cancer is likely to be the result of immunological tolerance within the tumor microenvironment, caused by various factors, including the presence of regulatory T cells, expression of CTLA-4 and PD-1, or downregulation of MHC class I on the cell surface [42]. Quantitative methods, including image analysis-directed rapid immuno-laser capture microdissection, will make it possible to analyze specific cell subsets isolated from specific histologic contexts [10] and to correlate them with the clinical outcome. In the end, systemic immune responses to viral proteins required for disease are weak, and do not reliably predict clinical behavior, underscoring the need for a better understanding of the mucosal microenvironment.
15.1.4 Preventive Vaccines
Current strategies for preventing HPV disease include screening, using either cytology or HPV testing, or a combination of both. Prophylactic vaccines that protect against infection with oncogenic HPV genotypes are comprised of noninfective recombinant virus-like particles (VLPs) of L1, one of the two HPV capsid proteins. These VLPs do not contain viral DNA and thus are completely noninfectious and non-oncogenic. Currently, three constructs are available: Cervarix©, which targets HPV types 16 and 18 (bHPV) and is administered with adjuvant ASO4; Gardasil4©, which targets HPV types 6, 11, 16, and 18 (qHPV); and Gardasil9©, which targets HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58 (nHPV) (Table 15.1). All formulations are highly effective against HPV infections, not only in the cervix but also in other anatomical sites, in both sexes [43, 44]. These vaccines elicit robust antibody responses that are 1–2 logs greater than those elicited by natural infection [25]. In persons known to have been previously exposed to HPV, a single vaccination with qHPV drastically enhanced both the magnitude and the quality of the antibody response. In contrast to the nonneutralizing antibodies generated by natural infections, antibodies elicited by vaccination were neutralizing [25]. While both vaccines bHPV and qHPV have demonstrated cross-protection to other HPV types [43, 45], Cervarix© provided cross-protection to HPVs 31, 33, 45, 51, and 52, while Gardasil4© has been shown to be cross-protective only to HPV 31. The broad cross-reactivity of Cervarix© is most likely attributable to its adjuvant, ASO4, which is a TLR4 agonist. The existing prophylactic vaccines, Gardasil© and Cervarix©, have no therapeutic effect. Nevertheless, emerging evidence from the study using quadrivalent HPV vaccine suggests that vaccination with a preventive vaccine after excisional treatment of CIN2/CIN3 significantly decreases the likelihood of disease recurrence [46, 47]. The mode of action of this protective effect is not known and is a subject of active investigation.
Vaccine | Manufacturer | HPV specificity | Adjuvant | Immune response |
|---|---|---|---|---|
Cervarix | GlaxoSmithKline | 16, 18 | AS04 (TLR4 agonist + aluminum salt | • Predominantly IgG1 |
• Cross-protection against HPVs 31, 33, 45, 51, and 52 | ||||
Gardasil4 | Merck | 6, 11, 16, 18 | Aluminum salt | • Cross-protection against HPV 31 |
Gardasil9 | Merck | 6, 11, 16, 18, 31, 33, 45, 52, 58 | Aluminum salt | • Not fully tested to date |
15.1.5 Therapeutic Vaccines
In contrast to the prophylactic vaccines, the development of new therapeutic vaccines is focused on targeting E6 and E7. Effector T cell responses to these viral, non-self-oncoproteins, which are constitutively expressed by transformed cells, are likely to play a role in mediating lesion regression. Persons with preinvasive disease present an unparalleled opportunity to determine proof of principle for immunotherapeutic strategies. These lesions are directly accessible and clinically indolent, providing an opportunity to assess the relevant tissue before and after intervention. Moreover, a subset of lesions do regress, thereby making it possible to determine either pretreatment characteristics that predict therapeutic effect or characteristics of induced immune responses that predict therapeutic benefit. Tissue studies will also afford the ability to determine mechanisms of immune suppression mediated by different stages of HPV disease.
Several vaccine platforms have been evaluated, including naked DNA administered intramuscularly (IM) [23, 48], DNA administered IM with electroporation [38, 49, 50], viral vectors including Modified Vaccinia Ankara (MVA) virus administered subcutaneously [51], vaccinia virus (TA-HPV) administered by scarification and by IM injection [52], peptides administered subcutaneously with adjuvant [53–55], and bacterial constructs such as Listeria monocytogenes [56]. Despite promising data derived from preclinical models, translation to humans has been modest. To date, vaccine-induced immune responses in humans with any stage of HPV disease have been minimal. In retrospect, this apparent discrepancy may be in part a consequence of trial design. Most trials administered immunotherapeutics peripherally [23, 38, 52], and most trials were designed to evaluate conventional, relatively binary endpoints, such as the magnitude of virus-specific CD8+ T cell immune responses in the blood and lesion regression versus persistence. In fact, in a Phase I clinical trial evaluation of heterologous DNA prime, recombinant vaccinia (TA-HPV) boost vaccination in persons with HPV 16+ CIN2/CIN3, prior to planned standard therapeutic resection, in persons who did have residual dysplasia at the tissue endpoint, post-vaccination tissue resection specimens showed drastic immunologic changes in the target lesions [57]. These included robust immune cell infiltrates in both the stromal and epithelial compartments, which were restricted to residual CIN2/CIN3, and did not involve immediately adjacent normal mucosa. These infiltrates were comprised of clonally expanded populations of T cell receptors, were in many cases organized into tertiary lymphoid structures or outright germinal centers, and had a TH-1 phenotype. This study established two critical points: that it was possible to elicit an effector response to antigens that had been present in a chronic fashion and that T cells generated by peripheral, intramuscular vaccination could traffic to the relevant immunologic target. In resections that had residual CIN2/CIN3, these lesions were heavily infiltrated with CD8+ T cells that were co-localized with apoptotic lesional squamous epithelial cells. This finding suggested that a planned resection proximate to vaccination essentially censored the tissue endpoint. By conventional measures, i.e., peripheral blood T cell responses to vaccine antigens, and complete histologic regression, this regimen was a failure. However, there was no way to conclude that vaccination had “failed,” given the findings in the target tissue. This insight has informed the design of subsequent clinical trials in persons with preinvasive HPV disease—tissue endpoints are obtained at a longer interval after therapeutic interventions. Although peripheral blood T cell responses are measured, quantitative measures in the lesion microenvironment are included.
A subsequent randomized placebo-controlled Phase II trial testing therapeutic vaccination for HPV 16 or 18+ CIN2/CIN3, using a synthetic DNA vaccine VGX-3100 administered IM followed by electroporation, reported a 49% rate of histologic regression in vaccinated subjects [38]. Although a subset of subjects who received placebo also had histologic regression, the rate of viral clearance was different; HPV became undetectable in 80% of vaccinated subjects who had histologic regression, in contrast to 30% of spontaneous regressions. The concomitant clearance of detectable virus in vaccinated subjects suggests that rates of recurrence may be lower in vaccinated subjects compared to those who received placebo; persistent HPV infection after resection of a preinvasive lesion is the most predictive risk for recurrence [58].
15.1.6 Adoptive T Cell Therapy
To date, no viable therapies have been identified for either metastatic or recurrent cancers. One approach that shows promise in patients with metastatic HPV disease involves identifying tumor-specific T cells from the endogenous response, namely, from tumor infiltrating lymphocytes (TILs). While some of the T cells in the tumor bed may be nonspecific, recruited by a chemokine gradient, in some solid tumors, many of the tumor T cells express activation markers that are upregulated when the cell is activated by engagement of the T cell receptor (TCR) with its cognate antigen. Two markers that have been investigated include PD-1 and CD137. Several groups have reported that activated T cells in the tumor microenvironment are enriched for clonally expanded populations of tumor-reactive T cells [59, 60]. The ability to isolate autologous HPV-specific TILs will provide the opportunity to assess the TCR repertoire and the functional polarization of relevant cells. A recent report of HPV-targeted TILs in persons with pretreated, metastatic cervical cancer describes tumor responses in three out of nine women, two complete responses and one partial response [61]. This outcome is significant because there are no effective therapies for either recurrent or metastatic cervical cancer or for any other metastatic HPV-attributable cancers. There is currently an ongoing clinical trial for treatment of metastatic HPV-associated cancers using tumor infiltrating lymphocytes (NCT01585428).
Another approach involves ex vivo stimulation of peripheral blood lymphocytes and infusion of antigen-specific T cells to the patient. Identification of TCRs with high avidity for tumor epitopes could pave the way to generate TCR libraries across HLA phenotypes. Analysis of the magnitude, breadth, and the quality of tissue TCRs is likely to yield insights to the successful cancer treatment modalities including adoptive T cell transfer. Autologous T cells could be genetically engineered to express HLA-matched HPV-specific TCRs and used as individualized treatment for HPV disease [60]. Currently ongoing clinical trials are investigating adoptive T cell therapies, including T cell receptor immunotherapy targeting HPV 16 E6 for HPV-associated cancers (NCT02280811) [62] and the use of HPV 16/18 E6-/E7-specific T lymphocytes, in relapsed HPV-associated cancers (NCT02379520).
15.1.6.1 Mechanisms of Immune Evasion in the Tumor Microenvironment
Like many other solid tumors, HPV-associated malignancies establish an immune-suppressive local microenvironment. The HPV life cycle is not cytolytic. Viral replication and assembly are temporally linked with cellular differentiation of squamous epithelial cells, and proinflammatory signals are absent [63]. Virus is shed in terminally differentiated squamous cells; thus, the initial exposure to HPV antigens is minimal and may not prompt robust activation of an immune response. Additionally, HPV downregulates cell surface MHC class I expression [64] and inhibits the production of proinflammatory cytokines [65–67]. E6 and E7 inhibit both interferon receptor signaling and activation of the interferon response genes [68]. E7 also further impairs the innate response by downregulating TLR9 transcription. Even incipient HPV disease recruits and functionally polarizes circulating monocytes, which migrate to the tumor site, where they differentiate into macrophages and dendritic cells [69].
Macrophage infiltration in both the stromal and epithelial compartments increases with the severity of HPV lesions, from HPV-infected cells to CIN2/CIN3 to squamous cancers [70, 71]. Their functional polarization to an immune-suppressive phenotype is mediated by tumor-secreted TGF-β and IL-10 [72]. As early in disease development as CIN2, the intensity of macrophage infiltrates correlates directly with the number of lymphatic vessels [71]. Epithelial expression of COX-2 increases with disease severity, which in turn inhibits dendritic cell (DC) maturation, reduces the ability of DCs to stimulate T cell proliferation, and increases production of IL-10 [73]. HPVs have also been shown to suppress maturation and function of Langerhans cells, which are epithelial-resident antigen-presenting cells [74–76]. However, this suppressive phenotype can be reversed in the presence of TLR3 agonists [77]. Finally, the tissue microenvironment induces and maintains tissue-specific gene expression and function of resident and recruited macrophages [78–80]. A growing body of evidence demonstrates functional plasticity in tissue macrophages; an induced suppressive or tolerizing phenotype can be reeducated by CD4+ TH1 T cells, to an activated, effector phenotype, with cell surface expression of costimulatory molecules [81].
Finally, individual cell subsets in HPV-associated malignancies, including tumor epithelium, tumor-associated macrophages, and CD8+ T cells, frequently express PD-L1, which is involved in normal immune homeostasis. Binding to its ligands, PD-1 and CD80, reduces CD8 T cell responses [82, 83]. While the presence of PD-L1 expression is associated with impaired cell-mediated immunity in HPV disease [83], tumor expression in and of itself is not a reliable biomarker for likelihood of response to PD-1 blockade [84]. The presence of a gene signature of interferon-γ-inducible genes, however, is associated with response to PD-1 blockade [85]. This finding is consistent with what has been observed in other solid tumors, namely, that clinical benefit from PD-1 blockade is more likely to occur in the setting of a preexisting host tumor-specific immune response [86]. Therefore, it is possible that an indirect measure such as Ki67, which is upregulated after activation via engagement of cognate antigen with the T cell receptor, may be more predictive of response.
15.1.7 Immune Checkpoint Inhibitors
One of the mechanisms by which tumors often suppress effector responses is by hijacking normal mechanisms of immune homeostasis. Under normal physiologic conditions, these mechanisms regulate the nature, quality, and duration of adaptive immune responses. Many malignancies essentially co-opt these homeostatic mechanisms to suppress effector T cell responses recruited to the tumor microenvironment. Therapies targeting ligand-receptor interactions of immune checkpoint pathways have been shown to be effective in enabling the function of immune effector responses in a subset of human cancers. One of the first immune checkpoint inhibitors tested in cancer immunotherapies was antibodies targeting CTLA-4, which is expressed on activated T cells. Blockade results in the broad enhancement of immune responses [87]. Clinical trials testing anti-CTLA-4 antibodies (ipilimumab), following standard of care chemoradiation in HPV-associated cancers, are ongoing in patients with advanced cervical cancer and head and neck cancers. The PD-1/PD-L1 pathway is another immune checkpoint that can be targeted by immunotherapeutic antibodies. PD-1 expression is upregulated on T cells after activation via recognition of cognate ligand. Expression is also upregulated by chronically stimulated T cells, in which it can reflect functional “exhaustion.” It is also present on B cells and NK cells [87]. Expression of one of its ligands, PD-L1, is induced by IFN-γ in the tumor microenvironment. Cell surface PD-L1 expression can be seen on the leading edge of tumor cells. Cytoplasmic expression has also been observed in both tumor cells and in tumor-associated macrophages [87]. Side effects associated with PD-1/PD-L1 blockade pathways have been considerably less severe than what has been observed with CTLA-4 blockade. Many anti-PD-1 (pembrolizumab and nivolumab) and anti-PD-L1 antibodies (durvalumab) are now being tested in clinical applications, either alone or in combination with conventional cancer treatment modalities, in patients with HPV-associated cancers. Similarly, interference with other immune checkpoint pathways, including OX40, 4-1BB, GITR, LAG-3, and HLA-E, is also being evaluated [62]. To date, across the board, approximately 20% of tumors respond to checkpoint blockade. The development of strategies to distinguish tumors that are likely to respond to blockade, versus tumors that are not, is an area of active investigation. Two clinical settings that are a focus of intense interest include tumors in which a preexisting antitumor response exists and tumors in which the mutational or neoantigen burden is high [42, 88].
15.1.8 Immunomodulatory Effects of Conventional Cancer Treatment Modalities
Conventional anticancer treatment modalities such as chemotherapy and radiation have immunomodulatory effects. Metronomic cyclophosphamide, for example, depletes circulating regulatory T cells (Tregs) [89]. The treatment options for inoperable primary tumors, and for recurrent and metastatic disease, include a combination of platinum-based chemotherapy and radiation, both of which are known to enhance susceptibility of cervical cancers to cytotoxic T cells, in addition to their direct cytotoxic effect [90]. Alkylating agents, including cisplatin, induce a high rate of genomic mutations [91]. Some of the immunomodulatory effects of radiation include upregulated expression of tumor-associated antigens [92] and upregulation of MHC class I expression on tumor cells. Ionizing radiation enhances epitope spreading [93]. An example of how these different attributes could be leveraged in persons with metastatic or recurrent disease might be to deplete Tregs with low-dose cyclophosphamide and prime initially with a therapeutic vaccine or infusion of tumor-specific CTL, followed by chemoradiation. In a recent report of the effect of a combination of carboplatin and paclitaxel (CarboTaxol) in patients with advanced cervical cancers, this regimen was followed by a significant decrease in the frequency of circulating myeloid cells, which reached nadir at 1–2 weeks after two cycles [94]. A second cohort then received a therapeutic vaccination 2 weeks after the last cycle of CarboTaxol. None of the patients had an endogenous, preexisting response to HPV 16. These responses were of greater magnitude than those observed in a previous trial in which patients were vaccinated 1 month after chemotherapy [95]. However, no clinical responses were reported. To date, no combinatorial immunotherapeutic trials have been carried out for cervical disease. However, in a recent report of pembrolizumab, an anti-PD-1 antibody, in patients with PD-L1-positive recurrent or metastatic squamous cancers of the head and neck, persons with HPV-associated disease had a 25% response rate. The expression of PD-L1 alone did not identify tumors likely to respond to PD-1 blockade. Rather, using a composite of PD-L1 expression on tumor cells and a gene expression signature of genes induced by IFN-γ identified a significant association with good response [84]. This finding is congruent with the observation that a preexisting CTL response to tumor was associated with better response to PD-1 blockade.
In sum, although much is known about the immunobiology of HPV-associated malignancies, there is much to learn about the timing and sequence of anticancer treatment modalities. HPV-associated lesions are relatively accessible, and viral antigens provide targets for both immunotherapy and for monitoring, and so it becomes possible to dissect out mechanisms of action in the lesion microenvironment, as well as identify proof of principle of therapeutic approaches.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree