Extensive colitis
Primary sclerosing cholangitis
Severe long-standing inflammation
Inflammatory polyps
Colonic stricture
Family history of colorectal cancer, especially aged <50
Personal history of dysplasia
Determination of anatomic extent in assessing cancer risk has historically been based on macroscopic rather than histologic inflammation. Both macroscopic and microscopic healing may occur, but once extensive colitis is documented, an increased risk of cancer should be assumed depending on the greatest previously determined extent. Mathy et al. [14] reported that colitis-associated cancer in patients with UC may arise in endoscopically normal but histologically involved areas of the colon. They concluded that further studies are needed to determine the risk of colitis-related neoplasia in patients with microscopic pancolitis but limited gross disease.
A study from the Netherlands suggested that cancers will be missed if surveillance is commenced at 8–10 years for patients with pancolitis and at 15–20 years for patients with left-sided disease because 9–15 % of the cancers that developed in their study patients occurred before these time frames [15]. Indeed, up to 22 % of patients who develop a colitis-associated colon carcinoma do so prior to commencing surveillance colonoscopies. However, the majority of studies have shown that the incidence is very low at 10 years of disease, and might even be decreasing [8, 16–18].
The wide variation of risk estimates reported in the literature may be attributed to differences in additional risk factors in the patient cohorts studied. The most consistent risk factor reported is primary sclerosing cholangitis (PSC), which corresponds to a CRC risk up to 31 % [19–21]. Soetikno et al. [22] conducted a meta-analysis of 11 studies and found that, overall, 21 % of the patients with both UC and PSC developed colorectal neoplasms, compared with 4 % of the patients without PSC.
Histologic or clinical disease activity is regarded as a risk factor for colitis-associated cancer [7, 23, 24]. Postinflammatory polyps may be markers of previous inflammatory severity and are closely related to the neoplasm [7, 25, 26]. A case-control study of patients with UC from St. Mark’s Hospital demonstrated that inflammatory polyps and colonic strictures increased the risk of CRC by twofold and fourfold, respectively, compared with individuals without these abnormalities [27]. In addition, a population-based cohort study reported that a family history of CRC was associated with a more than twofold higher risk of IBD-associated CRC [adjusted relative risk (RR) = 2.5; 95 % confidence interval (CI), 1.4–4.4]; furthermore, patients with a first-degree relative diagnosed with CRC before 50 years of age also had a higher risk (RR = 9.2; 95 % CI, 3.7–23) [28]. According to these data, the surveillance strategy for a particular patient should be determined based not only on the duration and extent of colitis but also on the presence of other risk factors.
4.3 Incidence and Prevalence of CD-Associated Cancer
Because of the chronic intestinal inflammation characteristic of the disease, CD is regarded as a risk factor for intestinal carcinoma. However, while some studies have reported that CD patients have an increased risk of cancer [29–35], other studies did not find any correlation [36–39]. In their 1994 study of the CRC risk in CD in a cohort of 281 patients, Gillen et al. [32] showed that those with extensive CD-associated colitis had an 18-fold higher risk, which decreased with increasing age at onset but increased significantly with a young age at onset.
A Canadian cohort study matched a population-based IBD database to a cancer registry in North America between 1984 and 1997 [34]. The incidence of CRC was higher in those with Crohn’s colitis (RR = 2.64; 95 % CI, 1.69–4.12) or UC (RR = 2.75; 95 % CI, 1.91–3.97) than in the general population. That study also found a higher risk of rectal cancer in patients with UC (RR = 1.90; 95 % CI, 1.05–3.43) but not in those with Crohn’s colitis (RR = 1.08; 95 % CI, 0.43–2.70).
Previous studies reported an increased risk of cancer in patients with CD of longer duration and extent [40, 41]. Stahl et al. [40] showed that patients with an early onset of CD were at higher risk for developing cancer; according to Maykel et al. [41], advanced age at CD diagnosis increased the risk of developing cancer [40, 41].
In the meta-analysis by von Roon et al. [42], based on 34 studies comprising 60,122 patients with CD, the RR of small bowel cancer or CRC compared with the baseline population was 28.4 (95 % CI, 14.46–55.66) and 2.4 (95 % CI, 1.56–4.36), respectively. In a subgroup analysis, patients with CD had an increased risk of colon cancer (RR = 2.59; 95 % CI, 1.54–4.36) but not of rectal cancer (RR = 1.46; 95 % CI, 0.8–2.55) [42].
The meta-analysis of Laukoetter et al. [43] consisted of 20 clinical studies with a total of 40,547 patients. The incidence of CD-associated cancer in CD patients was 0.8/1,000 person-years, meaning that during a 1-year observation period, 0.8 CD patients out of 1,000 developed CD-associated cancer. CD-associated CRC had a pooled incidence of 0.5/1,000 person-years (95 % CI, 0.3/1,000–0.6/1,000 person-years). The prevalence was 0.24 % (95 % CI, 0.19–0.28). The incidence of CRC and small bowel carcinoma in CD was 0.5/1,000 and 0.5/1,000 person-years, respectively. The pooled incidence of carcinomas in patients with fistulas was 0.2/1,000 person-years (95 % CI, 0.0/1,000–0.4/1,000 person-years). The mean duration between CD diagnosis and CD-associated cancer was 18.3 years. The authors concluded that although the risk of CRC is significantly increased in patients with CD, it is far lower than in those with long-standing UC. However, this meta-analysis did not find a significant relationship between cancer development and either the anatomic segments involved by CD or longer disease duration, although in the latter there was a trend toward a higher incidence. A stratification of cancer risk by disease duration has not been possible because of the paucity of appropriate studies [11].
Moreover, the benefit of surveillance colonoscopy in CD has yet to be established, and the early detection of small bowel carcinoma in patients with CD remains problematic. Routine magnetic resonance enteroclysis/enterography or capsule endoscopy could potentially detect these malignancies at an early stage, but the use of these methods to screen asymptomatic individuals is costly and has not been shown to prolong survival in patients with CD.
4.4 Timing of Initial Screening Colonoscopy and Surveillance Interval in Patients with UC (Table 4.2)
Most of the recent guidelines agree on the following: (1) For patients with proctosigmoiditis, regular screening or surveillance colonoscopy for detecting CRC is not necessary. (2) Surveillance colonoscopy should be started 6–10 years after the onset of symptoms for patients with left-sided or extensive colitis. (3) Ongoing surveillance colonoscopy should be carried out based on the individual risk profile.
Table 4.2
Comparison of screening recommendation from international guidelines for patients with colitis
ECCO 2013 | BSG 2010 | AGA 2010 | ACG 2010 | |
|---|---|---|---|---|
First screening | 6–8years | 10 years | Maximum 8 years | 8–10 years |
Surveillance interval | High risk, 1–2 years | High risk, 1 year | 1–3 years | 8–10 years |
Low risk, every 3–4 years | Intermediate risk, 3 years | More often at high risk | 1–2 years | |
E.g., PSC | ||||
Low risk, every 5 years | ||||
Biopsy | Targeted biopsy with chromoendoscopy by trained endoscopist | Targeted biopsy with chromoendoscopy is recommended. | Random biopsy (≥33) from each anatomic section | Multiple biopsies at regular intervals |
Quadrant biopsies every 10 cm | Two to four biopsies every 10 cm if no chromoendoscopy | Targeted biopsy with chromoendoscopy by trained endoscopist | Targeted biopsy with chromoendoscopy by trained endoscopist |
There are some minor variations among the guidelines with respect to the timing of initial screening colonoscopy and the surveillance interval. In the guidelines of both the ECCO and the BSG, initial screening and surveillance colonoscopy for detecting CRC are determined based on risk stratification. The 2013 guideline of the ECCO recommends that patients with extensive UC as well as those with left-sided UC who have at least 8 years of disease beginning from the onset of symptoms should undergo screening colonoscopy. The ECCO’s recommendations for surveillance colonoscopy are cited below [44]:
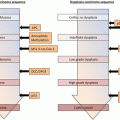
In the BSG guideline, the surveillance colonoscopy program is decided upon based on the individual risk profile (Fig. 4.1). Screening colonoscopy and surveillance colonoscopy are recommended as follows [45]:
According to the BSG, surveillance intervals are determined according to risk category (higher risk, yearly; moderate risk, every 3 years; higher risk, every 5 years).
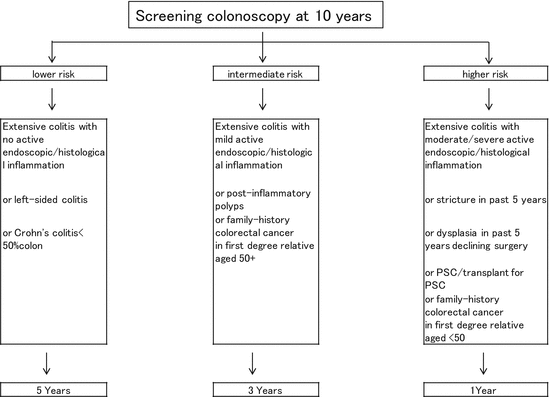
1.
In all patients with UC irrespective of the disease activity, a screening colonoscopy could be carried out 6–8 years after the beginning of symptoms to assess the patient’s individual risk profile.
2.
When disease activity is limited to the rectum without evidence of previous or current endoscopic and/or microscopic inflammation proximal to the rectum, inclusion in a regular surveillance colonoscopy program is not necessary.
3.
In cases with concurrent primary sclerosing cholangitis (PSC), surveillance colonoscopies should be carried out yearly from the point of PSC diagnosis irrespective of disease activity and extent.
4.
The CRC risk profile should be determined at the screening colonoscopy or the first surveillance colonoscopy 6–8 years after the first manifestation. Risk stratification mainly depends on extent of disease, severity endoscopic and/or histological inflammation, pseudopolyps, concurrence of PSC, and family history of CRC.
5.
The individual risk profile dictates surveillance colonoscopy intervals: every 1–2 years (high risk) or every 3–4 years (low risk) from the eighth year after the first manifestation in both extensive UC and left-sided UC.
1.
All patients with UC or Crohn’s colitis should undergo a screening colonoscopy approximately 10 years after the onset of colitis symptoms to assess disease extent and other endoscopic risk factors.
2.
Surveillance colonoscopies should be performed, where possible, when the disease is in remission. Surveillance procedure should not be unduly delayed if remission cannot be achieved.
3.
The risk of cancer is influenced by the duration and extent of disease and additional risk factors (such as PSC and family history of CRC) and is also linked to the endoscopic and histologic appearances at colonoscopy.
4.
If a dysplastic polyp is detected within an area of inflammation and can be removed in its entirety, it is not necessary to recommend colectomy.

Fig. 4.1
Surveillance recommendations for patients with colitis from the British Society of Gastroenterology
The 2010 guideline of the AGA recommends that all patients, regardless of the extent of disease at initial diagnosis, undergo a screening colonoscopy at a maximum of 8 years after the onset of symptoms [46]. The AGA’s recommendations for screening colonoscopy and surveillance colonoscopy are as follows:
1.
All patients, regardless of the extent of disease at initial diagnosis, should undergo a screening colonoscopy a maximum of 8 years after onset of symptoms, with multiple biopsy specimens obtained throughout the entire colon, to assess the true microscopic extent of inflammation.
2.
Patients with ulcerative proctitis or ulcerative proctosigmoiditis are not considered at increased risk for IBD-related CRC and thus may be managed on the basis of average-risk recommendations.
3.
Patients with extensive or left-sided colitis, with a negative screening colonoscopy, should begin regular surveillance colonoscopy within 1–2 years.
4.
After two negative examinations (no dysplasia or cancer), further surveillance examinations should be performed every 1–3 years. Increasing the frequency of surveillance colonoscopy to every 1–2 years after 20 years of disease is not needed for all patients but should be individualized according to the presence or absence of other risk factors.
5.
Patients with PSC should begin surveillance colonoscopy at the time of this diagnosis and then undergo yearly colonoscopy thereafter.
6.
Patients with a history of CRC in first-degree relatives, ongoing active endoscopic or histologic inflammation, or anatomic abnormalities such as a foreshortened colon, stricture, or multiple inflammatory pseudopolyps may benefit from more frequent surveillance examinations.
The 2010 American College of Gastroenterology guideline recommends that initial screening should be started after 8–10 years of colitis in patients with either left-sided colitis or pancolitis and annual or biannual surveillance should be performed [47].
The 2006 guideline for the management of UC in Japan recommends that surveillance colonoscopy should be started 8–10 years after disease onset for patients with extensive colitis and annual or biannual colonoscopy, including biopsies, beginning 8–10 years after disease onset in patients with extensive colitis [48]. In this guideline, surveillance colonoscopy for detecting CRC is not determined based on risk stratification.
4.5 Sampling Method: Step Biopsy or Target Biopsy? (Table 4.2)
While surveillance colonoscopy allows the early detection and treatment of CRC, especially in patients with long-standing UC, the flat or diffuse infiltrative macroscopic lesions that sometimes arise in UC are difficult to see endoscopically. In their 1986 study, Broström et al. [49] examined the use of surveillance colonoscopy for the early detection of dysplasia. The entire colon was separated into ten segments, and two biopsies were taken at each one. Manning et al. [50] reported the results of a prospective study of screening for colorectal epithelial dysplasia by regular colonoscopy. In their 1987 study, biopsy specimens were taken from flat mucosa at approximately 8–10-cm intervals along the colon and rectum and from any identifiable mass lesions. Based on these and other studies of surveillance colonoscopy, the practice of multiple biopsies in patients with long-standing UC during surveillance colonoscopy has been widely adopted.
Rubin et al. [51] used a mathematical model to show that 33 biopsy specimens were needed to detect dysplasia with 90 % probability, if dysplasia is present. At least 64 biopsy specimens were needed to reach a 95 % probability of detecting dysplasia. That study provided the basis for surveillance practice recommendations. Current guidelines for dysplasia surveillance recommend quadrant-based random biopsies every 10 cm throughout the entire colon and a minimum of 33 biopsies [44–47]. However, random biopsy samples only 0.03 % of the mucosal surface, has a detection rate of <2 per 1,000 biopsies [52], and does not affect clinical decision-making when advanced techniques are used [53]. Broek et al. [52] retrospectively analyzed 466 surveillance colonoscopies (in 167 patients) during which 11,772 random biopsies were obtained. The authors concluded that the low yield and lack of clinical consequences from random biopsies question its necessity and cost-effectiveness in UC surveillance. Thus, chromoendoscopy (CE) provides an alternative to colonoscopy with random biopsies, and its use is supported in recent guidelines [44, 45].
Moreover, recent data have shown that most gastroenterologists do not follow the recommended biopsy protocol. In a study from the Netherlands, only 27 % of gastroenterologists complied with the recommended number of 33 random biopsies [54]. Eaden et al. [55, 56] used a questionnaire to show that >50 % of the gastroenterologists surveyed obtained fewer than ten colonic mucosal biopsies per endoscopic surveillance examination.
The BSG guideline recommends pancolonic dye spraying with the targeted biopsy of abnormal areas; otherwise, two to four random biopsy specimens from every 10 cm of the colorectum should be taken, with additional biopsies of suspicious areas.
The Research Group of Intractable Inflammatory Bowel Disease of the Ministry of Health, Labour and Welfare of Japan is carrying out a randomized controlled study to compare the efficacy of step biopsy and target biopsy [57]. The results of this study will soon be available.
4.6 Chromoendoscopy and Other Newer Endoscopic Techniques
4.6.1 Chromoendoscopy
Target biopsy techniques have gained increasing acceptance [58]. Among these newer techniques CE is the most well established, and it has been used to better define the superficial gastrointestinal mucosa [59]. CE involves the topical application of a dye onto the colonic mucosa. It has two main advantages [27]: (1) it improves the detection of subtle colonic lesions, which raises the sensitivity of the endoscopic examination, and (2) once a lesion is detected, its CE appearance can aid in its characterization, which raises the specificity of the endoscopic examination [46, 56]. Previous studies demonstrated that the use of CE increases the detection rate of dysplasia by two- to threefold, corresponding to a per lesion increase of four- to fivefold [60–62].
Use of a magnifying colonoscope may further increase the sensitivity and specificity of CE [46]. Crypt architecture can be categorized by evaluating the pit pattern, which aides in the differentiation between neoplastic and nonneoplastic changes and in the performance of targeted biopsies [63].
The different stains used in CE can be classified as absorptive or contrast agents [59]. Absorptive agents include methylene blue (0.1–0.5 %) and cresyl violet (0.2 %). They are rapidly absorbed by normal colonic mucosa but poorly absorbed by dysplastic or inflamed tissue, thus enhancing the superficial structure of lesions and demonstrating the various cell types. Contrast agents, such as indigo carmine, pool in the mucosal grooves without reaction and absorption and thus highlight the superficial structure of lesions.
Several studies have evaluated the use of CE as an adjunctive method to diagnose dysplasia or cancer, based on its ability to more accurately evaluate the extent of disease and the degree of inflammatory activity [60, 62, 64–66]. In the study of Kiesslich et al. [64], patients with long-standing UC were assigned to conventional colonoscopy or colonoscopy with CE using methylene blue. The correlation between the endoscopic assessment of both the degree and the extent of colonic inflammation was better in the CE group than in patients examined with conventional colonoscopy. In addition, more targeted biopsies were possible with CE, and significantly more dysplasia was detected. The sensitivity and specificity for the differentiation of nonneoplastic from neoplastic lesions were both 93 %.
In a randomized control study, Rutter et al. [60] evaluated 100 patients with long-standing extensive UC who underwent “back to back” colonoscopies with both random and targeted biopsies, followed by spraying with indigo carmine and biopsies. Median extubation times for the first and second colonoscopies were 11 and 10 min, respectively. During conventional colonoscopy, 43 mucosal abnormalities were detected, of which two were dysplastic. Following dye spraying, 114 additional abnormalities were detected, of which seven were dysplastic. The targeted biopsy protocol detected dysplasia in significantly more patients than achieved with the nontargeted protocol [60].
Hurlstone et al. [66] analyzed 350 patients with long-standing UC who underwent surveillance colonoscopy using high-magnification CE. Quadrant-based biopsies at 10-cm intervals were taken on extubation, in addition to targeted biopsies of abnormal mucosal areas. The data were compared to those from 350 control patients, matched for disease duration and extent, who had undergone conventional colonoscopic surveillance. Significantly more intraepithelial neoplastic lesions were detected in the magnification chromoscopy group than in the controls (69 vs. 24 lesions, P < 0.0001). In addition, a greater number of flat lesions with intraepithelial neoplasia were detected in the CE group than in controls (53 vs. 14 lesions, P < 0.001). The authors concluded that magnification CE can predict neoplastic and nonneoplastic mucosal changes with a high overall accuracy [66].
4.6.2 Virtual Chromoendoscopy
Newer endoscopic techniques are being explored to aid in the diagnosis of dysplasia in IBD, although none has yet been rigorously studied. These new techniques include virtual chromoendoscopy (VCE), narrow band imaging (NBI) [67], fluorescence endoscopy [68, 69], optical coherence tomography [70], and confocal endomicroscopy [61, 70, 71].
VCE, also called dye-less chromoendoscopy, is a recently developed imaging technique that comprises NBI (Olympus, Tokyo, Japan), Fujinon intelligent color enhancement (FICE; Fujinon, Tokyo, Japan), and i-Scan (Pentax, Tokyo, Japan). NBI provides clear imaging of the microvascular structure of the colon. Its development was motivated by the quest for a simpler technique that would obviate the complexity of CE [67]. This unique form of electronic CE was first described by Gono et al. [72]. The method is based on illumination of the mucosal surface by light with a defined narrow band of wavelengths. Two narrow bands, centered at 415 (blue light) and 540 nm (green light), are used in the system introduced by Olympus (Olympus Medical, Tokyo, Japan). These selected wavelengths can pass through the mucosa to a defined depth, and their absorption correlates with the absorption maximum of a molecule of hemoglobin. A wavelength of 415 nm penetrates only the very superficial layers of the mucosa and is absorbed by blood in the intrapapillary capillary loops. The narrow band centered at 540 nm penetrates the deeper level and accentuates the venules and arterioles located under a layer of intrapapillary capillary loops. Using NBI to illuminate the mucosal membrane substantially increases the contrast between blood-containing vessels and the surrounding tissues, allowing even very subtle changes in the microvascular architecture to be identified [73].
Dekker et al. [67] carried out a prospective, randomized, crossover study of 42 patients with long-standing UC and compared the accuracy of NBI with that of standard colonoscopy for the detection of neoplasia. Using NBI, 52 visible lesions were identified in 17 patients, whereas with standard WLE, 28 lesions were detected in 13 patients. Pathologic evaluation of targeted biopsies identified 11 patients with neoplasia. Neoplasia was detected by both techniques in four patients but only by NBI or conventional colonoscopy in four and three patients, respectively. The sensitivity of that first-generation NBI system for the detection of neoplasia was comparable to that of conventional colonoscopy, although a larger number of suspicious lesions were found during NBI. The authors concluded that it is still too early to stop taking additional random biopsies at surveillance colonoscopy in patients with UC [67].
Two other trials demonstrated that high-definition WLE was equivalent to NBI [74, 75]. In the study of van den Broek et al. [74], 11 out of 16 (69 %) neoplastic lesions were detected by high-definition WLE and 13 out of 16 by NBI (81 %) (P = 0.727). In the study of Ignjatovic et al. [75], there was no difference in the proportion of patients with at least one area of dysplasia detected by WLE vs. by NBI, with five patients having at least one dysplastic lesion in each group [odds ratio (OR) = 1.00; 95 % CI, 0.27–3.67; P = 1.00]. This remained unchanged when adjusted for other variables (OR = 0.69; 95 % CI, 0.16–2.96; P = 0.62).
Efthymiou et al. [76] evaluated 44 patients with IBD to compare CE and NBI with respect to lesion detection and to assess the accuracy of the mucosal pit pattern (Kudo classification) as seen on NBI in predicting mucosal histology. CE identified more lesions than NBI (131 vs. 102, P < 0.001), detecting 23 neoplastic (dysplastic or indefinite for dysplasia) lesions in 11 patients compared with the 20 lesions in 10 patients detected by NBI (P = 0.180). Kudo classification by NBI had a low sensitivity and modest accuracy for dysplasia (42 % and 74 %, respectively). The authors concluded that there was a nonsignificant trend in favor of CE for the detection of dysplasia and that NBI cannot be recommended as an alternative to CE for dysplasia surveillance [76].
Pellisé et al. [77] examined the number of false-positive and true-positive lesions in patients undergoing CE and NBI as well as the proportion of patients with missed intraepithelial neoplasia lesions. In the per-lesion analysis, NBI had a significantly lower false-positive biopsy rate (P = 0.001) and a similar true-positive rate [77]. The efficacy of NBI in the endoscopy-based differential diagnosis of sporadic neoplasia vs. colitis-associated dysplasia or cancer remains to be confirmed [78].
4.6.3 Autofluorescence Imaging
Autofluorescence imaging (AFI) is a novel technique that takes advantage of the fluorescence of tissues exposed to ultraviolet (<400 nm) or short-wavelength visible (mostly blue) light. The autofluorescence produced by certain molecules (fluorophores) has a longer wavelength than that of the excitation light [59, 79]. Tissue autofluorescence is influenced by several factors, including the architecture and light absorption properties of the tissues (the latter is mainly determined by the concentration of hemoglobin in the tissues), their biochemical environment, and their metabolic status [59, 80]. These features are distinct in neoplasia, as the greenish background of normal colonic tissue is replaced by a purple-colored mucosa [59].
In their prospective pilot trial (n = 50) using a randomized crossover design between standard targeted WLE biopsies and AFI-targeted biopsies, van den Broek et al. [81] demonstrated significantly better detection in the AFI-first group, in which ten neoplastic lesions were detected among 25 patients, while WLE did not detect any additional lesions. In the WLE-first group, three lesions were detected among 25 patients, and subsequent AFI detected three more (P = 0.036). This study reported that the Kudo classification by NBI had a sensitivity and specificity of 75 % and 81 %, respectively, but on AFI all neoplasias were purple (sensitivity 100 %) [81].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree