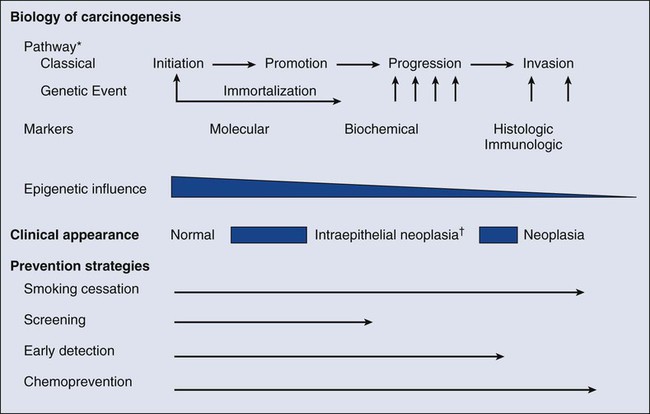
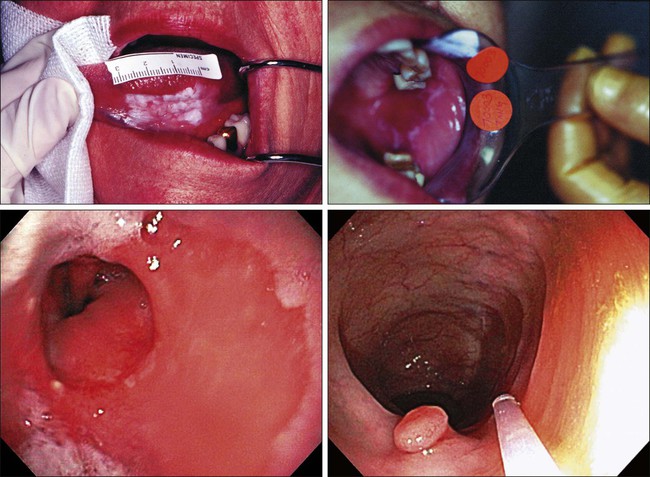
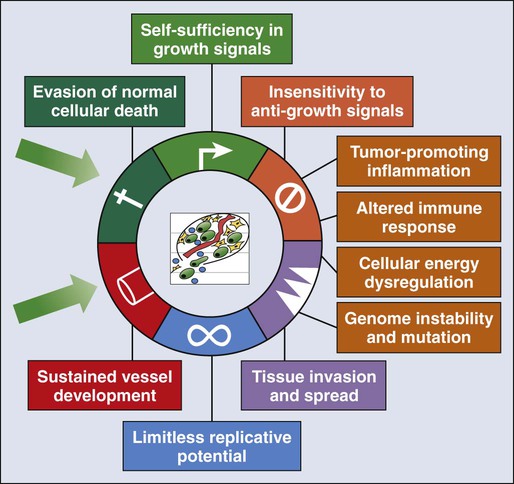
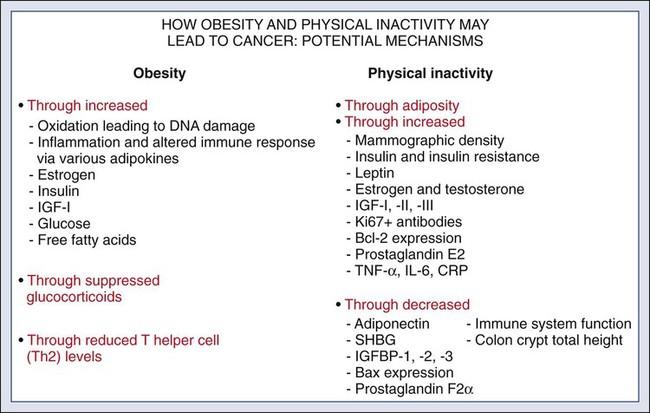
23 Therese B. Bevers, Powel H. Brown, Karen Colbert Maresso and Ernest T. Hawk • The six hallmarks, or cardinal derangements, characterizing all epithelial cancers are sustained proliferative signaling, evasion of growth suppressors, activation of invasion and metastasis, replicative immortality, induction of angiogenesis, and resisting cell death. These six hallmarks tend to occur in a permissive context characterized by four features: suppressed immune surveillance, tumor-promoting inflammation, cellular dysregulation, and genome instability and mutation. • Tobacco, which accounts for 30% of all cancers and 90% of lung cancers, is the greatest modifiable risk factor for cancer. The use of tobacco is on the rise in developing nations, and declines in smoking prevalence in the United States have recently begun to slow. Tobacco is likely to remain an important public health issue in the United States and globally for the foreseeable future unless tobacco control strategies can be more fully implemented and sustained. • After tobacco, obesity has the highest attributable cancer mortality. Recent evidence suggests that obesity, resulting at least in part from excess caloric intake, is a key driver in cancer development. Diet, physical inactivity, infections, and sun exposure contribute to cancer risk as well. • Data related to the role of nutrition in cancer risk is more persuasive for specific foods, rather than for specific nutrients or other food constituents. A few factors have been “convincingly” associated with an increased risk of cancer, as classified by the American Institute for Cancer Research, but none has been “convincingly” associated with a decreased risk. • Cancer incidence is set to double by 2030 as a result of a growing and aging population. • Nearly two thirds of all cancer deaths are attributable to tobacco, poor diet, physical inactivity, and obesity. • We can prevent approximately half of all cancers occurring today by implementing tools and knowledge we already have. • Thirteen chemopreventive agents have been approved by the U.S. Food and Drug Administration for treatment of precancerous lesions or to reduce the risk of invasive cancer, nearly all of which are for accessible organs. • The identification and eradication of a number of infectious, oncogenic agents can yield significant cancer preventive benefits as well. Globally, about 18% of cancers have an infectious etiology. In addition to the use of the human papillomavirus vaccines, vaccination for hepatitis B, “triple therapy” for Helicobacter pylori, and treatment of chronic hepatitis B and C can yield a marked reduction in the cancer burden in regions where these agents are endemic, although these medical interventions are not labeled for a cancer preventive indication. • Because chemoprevention focuses on healthy individuals in whom cancer may never develop, balancing the risks and benefits of any chemopreventive intervention is central to their development. • In the future, chemopreventive trials must be smaller, faster, cheaper, and focused on high-risk cohorts and drug combinations. Integrative assessments of the full range of benefits and risks of chemopreventive agents across cancer sites and diseases will also be important. • Population-based screening tests are available for the following cancers: cervical (Papanicolaou test), colon (colonoscopy, fecal occult blood testing, flexible sigmoidoscopy, and double-contrast barium enema), breast (mammography), and prostate (prostate-specific antigen test). • The National Lung Screening Trial study demonstrated that low-dose helical computed tomography screening can reduce lung cancer mortality by 20%. • Numerous genomics-based and proteomics-based approaches are attempting to identify biomarkers that can aid in the risk assessment and early detection of various cancers. The International Agency for Research on Cancer (IARC) estimates a doubling of cancer incidence worldwide by 2030 because of a growing and aging population.1 Nearly two thirds of all cancer deaths are attributable to the modifiable risk factors of tobacco, poor diet, physical inactivity, and obesity.2 Although rates of these risk factors have slowed or leveled off within the United States, they are increasing in many parts of the world, particularly as persons in developing countries adopt westernized lifestyles. Such trends are not sustainable. Efforts directed toward reducing these risk factors and improving rates of screening in the population can substantially mitigate the cancer burden. Within the clinic, enhanced risk stratification and novel preventive strategies targeted toward high-risk groups are needed. In sum, the need for safe and effective preventive strategies has never been more urgent. Carcinogenesis, a multistep, chronic process occurring over decades, is characterized by a progression of genetic changes affecting cellular identity and growth that ultimately culminates in cancer (Fig. 23-1). Our understanding of this process has emerged from the study of cancer development in carcinogen-driven or genetically driven animal models and human specimens. Conceptually, carcinogenesis can be thought of as occurring in three steps: initiation, which is the irreversible alteration of a cancer-related gene, such as an oncogene or tumor suppressor gene; promotion, the clonal expansion of the initiated cell, which is reversible if detected; and progression, the final stage, which is characterized by the transformation of a benign mass of cells into a malignant tumor, driven by the acquisition of additional mutations. The order of acquired mutations in carcinogenesis is not as important as their overall accumulation, and although the particular affected pathway(s) and acquired mutations vary across the different cancer sites, the paradigm of carcinogenesis and the six typical cardinal derangements (i.e., evasion of cell death, self-sufficiency in growth signals, insensitivity to antigrowth signals, tissue invasion and spread, limitless replicative potential, and sustained vessel development) and four contextual influences (i.e., tumor-promoting inflammation, altered immune response, cellular energy dysregulation, and genome instability and mutation) characterizing epithelial cancers are universal (Fig. 23-2).3 The cumulative genetic and epigenetic aberrations are typically reflected in a series of cytomorphologic and histopathological derangements termed “preinvasive neoplastic lesions,” or more simply, precancerous lesions (Fig. 23-3). These lesions are characterized by seven morphologic features: increased nuclear size, abnormal nuclear shape, increased nuclear stain uptake, nuclear pleomorphism (i.e., increased cell-to-cell variation in size, shape, and staining), increased mitoses, abnormal mitoses, and disordered or absent differentiation.4 A number of risk prediction models have been developed during the past 10 to 20 years in an effort to more accurately and reliably assess cancer risks in average- and high-risk individuals. These models are important as a foundation of risk : benefit discussions between a practitioner and patient and also to aid research and developmental clinical trials in support of both devices and interventions. The National Cancer Institute (NCI) maintains an important bibliography of peer-reviewed models relating to both risk of gene carrier status and absolute cancer risk prediction, as well as online risk prediction tools and calculators, on its Web site at http://epi.grants.cancer.gov/cancer_risk_prediction/. Although the number of new cancer cases is expected to rise by more than 50% within the United States and more than double worldwide by 2030,1 it is estimated that more than half of all cancers can be prevented by applying what we already know.5 In 1981 Doll and Peto6 published their landmark estimates of the relative contributions of the avoidable causes of cancer and concluded that up to three fourths of all cancers occurring in the United States during 1970 theoretically may have been avoided. This estimate as to the “preventability” of cancer was based on their approximations of the relative contribution of individual cancer risk factors: 30% for tobacco, 2% to 4% for alcohol, 35% for diet, 1% to 10% for infectious agents, 2% for pollution, and 4% for occupational exposures.6 Subsequent epidemiological data have largely supported these estimates and have identified obesity and lack of physical activity as additional cancer risk factors, causing a slight redistribution of the percentage of cancer mortality attributable to the various lifestyle risk factors. A review summarizing more recent data on this topic states that obesity accounts for 15% to 20% of cancers, physical inactivity accounts for approximately 5%, and the estimates of diet have decreased to 5%, much less than the initial estimate of 35% by Doll and Peto (Table 23-1).2 Although there are substantial limitations to estimates of the contributions of individual risk factors to cancer mortality, and although the estimated fraction of cancer that can be prevented may be a best-case scenario that is difficult to achieve in reality, such studies not only demonstrate a need to better understand the interplay among lifestyle factors but highlight the potential impact of prevention and underscore its urgency. Table 23-1 Relative Contribution of Individual Cancer Risk Factors to Overall Cancer Mortality Data from Colditz GA, Wei EK. Preventability of cancer: the relative contributions of biologic and social and physical environmental determinants of cancer mortality. Annu Rev Public Health 2012;33:137–56. Tobacco accounts for approximately one third of all cancer deaths, more than any other risk factor. In the United States, although the prevalence of smoking has been declining for decades, this decline has recently begun to slow and has not been observed in all population subgroups.7 Based on such data, the Centers for Disease Control and Prevention (CDC) state that tobacco is likely to remain an important public health issue in the United States for the foreseeable future unless tobacco control strategies can be more fully implemented and sustained.8 Some recent federal tobacco control initiatives include provisions in the Affordable Care Act (Table 23-2) that allow for coverage of evidence-based smoking cessation treatments and the Family Smoking Prevention and Tobacco Control Act, which grants the U.S. Food and Drug Administration (FDA) the authority to regulate the manufacturing, sale, and marketing of tobacco products. Worldwide, tobacco use is on the rise in developing nations, which harbor a majority of the world’s population, and where tobacco-attributable deaths are expected to significantly increase by 2050.9,10 In an effort to combat global tobacco use, the World Health Organization’s Framework Convention on Tobacco Control, the first international public health treaty, outlines tobacco control and regulation strategies for ratifying countries.11 Table 23-2 Affordable Care Act Provisions that Advance Cancer Prevention in the Public Data from Preston CM, Alexander M. Prevention in the United States Affordable Care Act. J Prev Med Public Health 2010;43:455–8. According to the most recent data, obesity is now the risk factor with the highest attributable cancer mortality (14% to 20%) after tobacco.2,12 During the past 30 years, the United States has experienced an epidemic of obesity, with its prevalence more than doubling between the periods of 1976-1980 and 2003-2006.13 In 2011, nearly two thirds of the U.S. population was overweight or obese, and higher prevalences are observed in various subgroups, such as racial and ethnic minorities, where the proportion of overweight or obese individuals can exceed 70%.14 The United States is not alone in this epidemic, as other developed nations have also experienced increasing proportions of overweight and obese individuals.13 The mechanisms of how excess body weight influences cancer risk and progression is an area of active research. The strongest empirical data suggest that this increased risk may, at least in part, be mediated by the effect of the various peptides and steroid hormones produced by adipose tissue on other cell types along the neoplastic continuum (Fig. 23-4).13,15 Overweight and obesity have now been definitively associated with an increased risk of six cancers—postmenopausal breast, colorectal, esophagus, pancreas, endometrial, and kidney—and possibly others, including prostate and ovarian.16 In addition, higher body mass index (BMI) has been associated with increased death rates in breast, endometrial, colorectal, esophageal, pancreatic, kidney, gastric, cervical, uterine, ovarian, and prostate cancers; multiple myeloma; and non-Hodgkin lymphoma.12,17,18 American Cancer Society (ACS) recommendations that affect this modifiable risk factor include balancing caloric intake with physical activity, avoiding excessive weight gain throughout life, and, if overweight or obese, achieving and maintaining a healthy weight.19 Evidence for the protective effect of physical activity with regard to cancer risk continues to grow. It is important to note that physical activity has independent protective effects beyond its indirect effect on cancer risk through body weight. The protective effect of physical activity has been most convincingly established for breast and colorectal cancers, but some studies have also demonstrated similar effects on endometrial and lung cancers.20 As with body weight, the effects of physical activity are not limited to the development of cancer but may be observed across the cancer continuum, with some studies suggesting a beneficial effect on survival in patients with breast and colorectal cancers.17,21–24 Although the exact mechanisms of the protective effects of physical activity on cancer are unclear, current evidence suggests that they may result from reductions in circulating concentrations of various hormones and by enhancements to overall energy metabolism (Fig. 23-4).20,25 Recommendations from the ACS relating to physical activity include getting 150 minutes of moderate-intensity or 75 minutes of vigorous-intensity physical activity each week and limiting sedentary behavior.19 Independent of obesity, certain foods, drinks, vitamins or other nutrients, and particular dietary patterns may increase or decrease the risk of various cancers. The association of diet with risk of cancer has been extensively studied, although few specific dietary components have been convincingly shown to either increase or decrease risk. This situation is due in large part to challenges in the accuracy and reliability of long-term dietary measurements and the likely long-term follow-up required to observe effects of any single dietary component, combination, or pattern. Most recently, in the first study to examine multivitamin use over several years of follow-up in a large cohort, Gaziano et al.26 report that daily multivitamin use among male physicians over a median of 11.2 years of follow-up modestly but significantly reduced the risk of total cancer (hazard ratio [HR], 0.92; 95% confidence interval [CI], 0.86-0.998; P = .04).26 Although there is no single ideal way to categorize the evidence on food and nutrition, the World Cancer Research Fund/American Institute for Cancer Research publishes the most comprehensive and systematic assessment of epidemiological evidence regarding diet in cancer prevention.16 Based on this source, a few generalizations can be made. First, the evidence is more persuasive for specific foods rather than for specific nutrients or food constituents. Second, although a few factors have been “convincingly” associated with an increased risk of cancer, none has been “convincingly” associated with a decreased risk. Foods and particular food constituents with evidence for increasing cancer risk deemed as “convincing” by the American Institute for Cancer Research include aflatoxins in persons with liver cancer, beta-carotene supplements in smokers and arsenic in drinking water for persons with lung cancer, red or processed meats in persons with colorectal cancer, and alcoholic drinks in persons with colorectal, esophageal, oropharynx, and both pre- and postmenopausal breast cancers.16 Although currently no evidence exists for any food, dietary constituent, or pattern deemed as “convincing” for decreasing cancer risk, nonstarchy vegetables and fruits display the most consistent evidence, deemed as “probable,” for a protective effect on the risk of oropharynx, esophageal, stomach, and lung cancers.16 In addition, garlic, milk, calcium, and foods containing fiber demonstrate “probable” evidence for decreasing the risk of colorectal cancer, as does folate for pancreatic cancer and lycopene, selenium, and diets high in calcium for prostate cancer.16 Based on these findings, the ACS recommends a diet based on plant foods and whole grains, with limited intake of red or processed meats and alcohol.19 Infectious agents are an additional root cause of cancer and represent a significant cause of cancer in the developing world, where the medical care and resources necessary to prevent and control their transmission are scarce. Worldwide, it is estimated that approximately 18% of cancers have an infectious etiology.27 Adequate prevention or treatment of infections may be associated with a substantially reduced risk of several cancers, including liver (hepatitis B and C); cervical, oral, anal, and head and neck (human papillomavirus [HPV]); Kaposi sarcoma and non-Hodgkin lymphoma (human immunodeficiency virus [HIV]/AIDS); bladder cancer (schistosomiasis); gastric cancer (Helicobacter pylori); nasopharyngeal cancer (Epstein-Barr virus [EBV]); and adult T-cell leukemia/lymphoma (human T-cell lymphotropic virus–1). Currently, the only therapies for treating infectious agents that have been formally FDA-approved with the indication of cancer risk reduction are the HPV vaccines Gardasil, approved in 2006, and Cervarix, approved in 2009, although hepatitis B vaccine and treatment of hepatitis C, HIV, H. pylori, and schistosomiasis infections are believed, if not actually demonstrated, to result in cancer risk reductions as well (Table 23-3). Table 23-3 Strategies for Probable Cancer Risk Reduction in the Setting of an Infectious Agent Sun and radiation exposure, the final modifiable risk factors, are estimated to account for 2% of preventable cancers.2 Skin cancer is the most common form of cancer, and its incidence is highest among non-Hispanic white persons. In recent years, the incidence of skin cancer has been rising, particularly for melanoma among white persons, for whom incidence has been increasing by 3% annually since 2004.7 Some possible factors contributing to this rise include a decrease in the protective ozone layer, changing patterns of dress favoring more skin exposure, more leisure time spent in the sun, and increased use of tanning beds.28 Prevention of skin cancer is dependent upon “sun-protective” behaviors, including covering up skin or avoiding the sun during its peak hours, seeking shade, applying sunscreen, and avoiding use of tanning beds.28 • an abnormal level of expression in early, preinvasive human neoplasia relative to the normal epithelium from which such lesions are derived; • a clear biological contribution to the initiation, maintenance, or progression of a preinvasive neoplasm in a model system rather than just a bystander role; • pharmacologic accessibility, with its modulation resulting in reductions in neoplastic incidence, histopathological grade, cancer mortality, and/or other convincing pathological evidence of improvement; and finally, • specificity for neoplasia, rather than the normal tissue from which cancers arise. Although use of the term “chemoprevention” is generally limited to the oncology field, it is nevertheless a well-established concept in both chronic and infectious disease prevention. The use of lipid-lowering and antihypertensive medications to reduce the risk of coronary artery disease, myocardial infarction, and stroke or the use of vaccines and various antibiotics to prevent infectious diseases are excellent examples of effective “chemoprevention” regimens that are well established and well accepted in clinical medicine. A major hurdle to the establishment and acceptance of chemoprevention within cancer medicine relates to a lack of accurate, reliable, and serially accessible surrogate end point biomarkers (SEBs) that reliably correlate with risk of disease.4 Biomarkers that occur early in the carcinogenic process and that can substitute for the true end point of cancer incidence or mortality—in that they strongly correlate with their risk—are urgently needed. Much as blood pressure and low-density lipoprotein cholesterol levels predict the risk of cardiovascular and cerebrovascular diseases and have served as focal points of preventive efforts in cardiovascular medicine, clinical oncology still requires these types of biomarkers that correlate with risk and that can serve as targets for cancer preventive efforts. Despite this challenge, we have seen some success in the setting of intraepithelial neoplasia when it is used as an SEB. A number of potential chemopreventive agents have entered clinical trials, and 13 have received FDA approval specifically for treatment of precancerous lesions or to reduce the risk of invasive cancer (Table 23-4).29 An evaluation of the end points in clinical trials that have resulted in the regulatory approval of these preventive agents reveals that nearly all have been approved for indications relating to intraepithelial neoplasia treatment, particularly in accessible organs, as opposed to prevention, per se. Table 23-4 Approved Agents for Treatment of Precancerous Lesions or Cancer Risk Reduction, 2012 *Food and Drug Administration labeling voluntarily withdrawn by Pfizer, February 2011. The development of chemopreventive compounds proceeds through a phased process of clinical trials akin to other areas of therapeutic development, but with somewhat greater attention to safety (Table 23-5).30 Phase I studies are designed to quickly evaluate an acceptable dose or dose range in a small number of healthy volunteers, whereas phase II trials generate preliminary, short-term efficacy data based on changes in any number of biomarkers believed to relate to cancer risk and confirm safety data in the context of a specific neoplastic condition. Phase II trials can be divided into phase IIa and phase IIb, where phase IIa trials establish preliminary drug effects in an uncontrolled manner and phase IIb trials compare the experimental agent with a placebo or the standard of care against scientifically or clinically justified biomarkers of neoplasia. Phase III trials typically involve hundreds to thousands of carefully selected subjects observed over years and typically randomly assign participants to a preventive agent or agent combination versus placebo or the standard of care to reduce clinically relevant neoplasia. Phase IV trials are undertaken after the drug has been approved by the FDA and marketed. The goal of this phase is to monitor the long-term safety and effectiveness of the agent or agent combination in a less controlled and more clinically relevant, “real-world” setting. Table 23-5 Characteristics of Clinical Trial Phases *In cancer chemoprevention, placebo may represent the standard of care. Reprinted from Viner JL, Richmond E, Hawk ET, Lawrence J. Chemoprevention: the cancer handbook. Hoboken, NJ: John Wiley & Sons; 2005. Central to the development of chemopreventive agents is the imperative to balance risks and benefits of an applied intervention, because “healthy,” or at least asymptomatic, populations are the focus of chemoprevention, thus necessitating a higher level of vigilance against potential toxicities and harms associated with an agent. The FDA has stated that because strategies to prevent cancer expose healthy people in whom cancer may never develop to a drug and its potential harms, a high level of certainty associated with benefits and harms of any potential intervention must exist.31 Consequently, the need exists to find better ways to balance risks and benefits to achieve acceptable therapeutic indices of chemopreventive agents. In this regard, agents need to be prioritized and tested with more attention to the relevance of model systems to the clinical context in which agents will be placed and their proposed mechanism(s) of action. Additionally, given that chemopreventive agents are often administered orally, yielding systemic exposure with the potential for broad efficacy across organs, integrative assessments of the risks and benefits of agents across multiple cancers and multiple diseases (e.g., cancer and cardiovascular disease) may be needed to tip the risk-benefit ratio in favor of their acceptance. In conclusion, chemoprevention represents an important part of the future of cancer medicine, and the identification of chemopreventive agents holds tremendous promise in reducing the burden of cancer. Challenges facing the field that will significantly affect its future development relate to the need for early-stage biomarkers and acceptable SEBs and the need to improve upon the risk-benefit ratio of agents. Trials must be smaller, faster, cheaper, and focused on high-risk cohorts, as such cohorts that offer more power over a shorter time frame, are more likely to be tolerant of adverse effects, and have more motivation to adhere to a given intervention. In addition, the pharmaceutical industry is faced with many business disincentives when it comes to investing in cancer prevention; consequently, new business models and incentives are needed to stimulate private investment and motivate pharmaceutical companies to truly invest in chemoprevention.32 Finally, there is a strong interest in novel therapeutic approaches such as synthetic lethality, which targets early molecular derangements in cancer development,33 and chemopreventive combinations such as the coadministration of eflornithine and sulindac, which demonstrates that synergy between agents can lead to improved efficacy (up to 70% to 90% reductions in recurrent precancerous lesions) at lower doses, resulting in fewer and/or less severe toxicities.34 Technological advances in screening and early detection are essential for progress in both the prevention and treatment of cancer, but incorporation of such advances into useful clinical practice is often challenging and requires careful consideration of all potential risks and benefits. For any screening test to be useful, three criteria must be met: first, a test must exist that will detect the disease earlier than routine methods; second, evidence must exist that earlier treatment leads to improved outcomes; and third, benefits of screening must outweigh the risks associated with any subsequent diagnostic and therapeutic treatments. Observational data may suggest the benefits of screening tests, but they have the potential to mislead as well as to inform because of at least two important potential biases: lead-time and length biases (Fig. 23-5). Because the intent of screening is to advance the date of diagnosis, lead time refers to the amount of time between screen-detected versus symptom-detected diagnosis. This lead time in diagnosis appears to prolong survival in screened individuals, although mortality in this group may not actually be delayed, creating a lead-time bias.35 Length bias refers to the tendency of screening to detect cancers that are indolent and slower growing because of their longer detectable preclinical phase compared with faster-growing, more aggressive forms of cancer. Consequently, in a group of screened individuals, this phenomenon creates the appearance that screening is extending survival, when in fact extended survival is due to the more indolent nature of the cancers found in this group and not necessarily to the screening itself.35 To avoid these biases, development of effective screening tests should culminate in well-conceived and well-conducted randomized, controlled trials assessing a mortality end point. In any situation in which a screenable disease is prevalent but potentially indolent, the potential exists for overscreening, overdiagnosis, and overtreatment, resulting in a range of personal and social costs that may ultimately outweigh the intended benefits. According to Croswell et al.,35 overdiagnosis is an extreme form of length-biased sampling and occurs when a lesion is diagnosed that would otherwise never have caused symptoms. Overscreening refers to the same phenomenon, where cancers are detected by screening that would otherwise never go on to cause problems for the individual. An excellent example of these issues that challenge screening efforts involves the use of the prostate-specific antigen (PSA) test for prostate cancer screening, wherein enthusiasm for PSA testing to reduce cancer mortality appears to have exceeded the actual data. In addition, the routine use of screening mammography in women between the ages of 40 and 50 years of age has become a topic of debate, given concerns over the ability of the test to detect lesions (i.e., ductal carcinoma in situ) that may never progress to invasive cancer. Such issues have challenged the overall science of screening and have led to an increased reliance on disease-specific versus all-cause mortality and risk-benefit measures within screening trials.36,37 Currently, population-based screening tests are available for the following cancers: cervical (Papanicolaou [Pap] test), colon (colonoscopy, fecal occult blood testing [FOBT], flexible sigmoidoscopy, and double-contrast barium enema), breast (mammography), and prostate (PSA test). However, based on systematic reviews, only tests for breast, cervical, and colon cancers are currently recommended in average-risk populations by the U.S. Preventive Services Task Force (USPSTF) (Table 23-6). An examination of Behavioral Risk Factor Surveillance System data from recent years demonstrates that mammography use has not substantially changed since 2000, with 75% of women aged 40 years or older reporting having had a mammogram with the past 2 years.38 However, this same study documented that colorectal cancer screening rates continue to lag behind those of other cancer screening tests, with 65% of persons aged 50 to 75 years reporting having had one of the screening tests recommended by the USPSTF.39 Various research efforts are directed at making more cancers amenable to screening, including the application of novel imaging modalities and the development of early-stage biomarkers. Research into imaging modalities has advanced in the area of lung cancer, where low-dose helical computed tomography (CT) screening has been recently shown to significantly reduce lung cancer mortality by 20%.40 In addition, numerous genomics- and proteomics-based approaches are attempting to identify biomarkers that can aid in the risk assessment and early detection of various cancers, such as recent proteomic work to identify plasma antibodies predictive of triple-negative breast cancer41 and the recent mapping of colorectal susceptibility loci through genome-wide association studies.42 Although we have established screening tools for some cancers—and aside from the challenges associated with the development and implementation of new tests for others—a significant challenge to the field will be to apply all recommended screening tests in an equitable manner to all persons who need them. Table 23-6 Most Recent United States Preventive Services Task Force Classification of the Evidence for Various Cancer Screening Tests in Average-Risk Individuals* Colon: CT colonography, fecal DNA testing (both are recommended by ACS as screening tests for colorectal cancer) Lung: Low-dose computerized tomography, chest x-ray, sputum cytology, or a combination of these Skin¶: Whole-body skin examination by a primary-care physician or patient skin self-examination *Each cell within the table corresponds to a USPSTF recommendation grade, which is based on the magnitude and certainty of the net benefit deriving from the screening test. Only the tests receiving an “A” or “B” classification are recommended to the general public and are reimbursed by Medicare under the Affordable Care Act. †ACS recommendations for colorectal screening also include CT colonography and fecal DNA testing, in addition to FOBT, flexible sigmoidoscopy, and colonoscopy. ‡ACS recommends annual mammography for all women 40+ years. §ACS recommends PSA testing for men 50+ after careful discussion of benefits and harms of test. This discussion should occur at age 45 years for high-risk men. ||The USPSTF recommendation regarding prostate cancer screening does not apply to PSA testing as part of surveillance after diagnosis or treatment of prostate cancer. ¶Recommended by the ACS as part of a periodic cancer-related check-up. Lung cancer is the most common cause of cancer-related death and the second most diagnosed cancer in the United States for both men and women. Estimated figures for 2012 alone exceed 225,000 new cases and 160,000 deaths, with men carrying roughly a 5% greater risk than women, and African American men at highest risk.7 On a global scale, these figures escalate, with lung cancer leading both worldwide cancer cases (more than 1.3 million diagnoses) and cancer-related mortality.45–45 Although American lung cancer incidence and death rates have been declining in men for more than a decade, the first statistically significant decrease in these rates among women was recently reported for the 2003-2007 period, after several decades of successive increases in incidence and death rates among women46 (see Chapter 72). As the major cause of lung cancer in the United States, smoking contributes to lung cancer–related deaths at a rate of approximately 90% in men and 80% in women,47,48 with the variation due to gender-biased decades of peak cigarette use.46 Additional risk factors associated with smoking include (1) amount smoked (pack-years), (2) exposure to secondhand smoke, (3) age at smoking onset, (4) type of product smoked (e.g., filtered/unfiltered and tar/nicotine content), and (5) depth of inhalation. In addition to tobacco, other risk factors include exposure to alternative toxins, such as asbestos, metals, radon, air pollution, or paint, which can be exacerbated by a family history of lung cancer or tobacco exposure.7,49 Current risk assessment for lung cancer is based primarily on smoking history, and although established clinical applications of risk modeling have existed for more than a decade for breast cancer50,51 and cardiovascular disease,54–54 models assessing risk of lung cancer have only recently been developed, and include Bach, Spitz, Liverpool Lung Project, and van Klaveren risk models.55–60 These models, based on epidemiological risk factors alone or in combination with DNA repair biomarkers,59 have been designed for limited use in the general60 and high-risk55 lung cancer populations. The Bach model was initially developed in 2003 based on data from 18,172 participants of the Carotene and Retinol Efficacy Trial (CARET).55,57 Model inputs include basic demographic and exposure data from each participant (i.e., age, sex, exposure history to asbestos, and smoking history, including duration and packs per day and/or duration of abstinence), and the model estimates individual absolute risk of diagnosis of lung cancer within 10 years. Spitz et al.61 then constructed and validated individual lung cancer risk models for current and former smokers and persons who never smoked using epidemiological data from 1851 patients with lung cancer at MD Anderson Cancer Center and 2001 matched control subjects.61 The model’s predictive precision was subsequently improved by incorporating two biomarkers for DNA repair,59 leading to improved concordance statistics of 0.73 (current smokers) and 0.70 (former smokers). Like the Spitz model, the Liverpool Lung Project model originated as an epidemiological model based on five risk factors and DNA genotyping data, including smoking duration, prior diagnosis of pneumonia, occupational exposure to asbestos, prior diagnosis of malignant tumor, family history of lung cancer, and a single-nucleotide polymorphism (SNP) within the seizure 6–like (SEZ6L) gene.56,62 In an effort to develop a more comprehensive risk assessment model for lung cancer, Maisonneuve et al.58 investigated another strategy that combines both epidemiological and clinical risk factors. By using data from the COSMOS trial to recalibrate the Bach model and then incorporating low-dose CT (LD-CT) findings at baseline screening, these investigators have identified a potential model that assists with determination of the time interval to subsequent screening for persons in a high-risk lung cancer population. Furthermore, this model appears to stratify individuals according to their risk of lung cancer diagnosis at repeat screening scans. Although these results exhibit the potential to support large-scale screening programs, validation of these results remains to be proved. With the more recent data concerning risk of cancer, both biological and genetic, lung cancer risk models are currently heading toward an integrative strategy incorporating epidemiological risk factors with clinical risk factors.63,64 Ultimately, by adding to this bilateral approach the additional aspects of molecular epidemiology (e.g., SNPs and genome-wide studies) and both lung and nonlung biomarker assessments, lung cancer risk assessment will take its next step.63 Lung cancer primary prevention strategies include smoking avoidance and cessation and eliminating exposure to other toxins, such as asbestos and radon. In support of this, alternative nicotine replacement therapy (NRT) approaches have been developed, including the nicotine patch, nicotine gum, and nicotine inhalers. These therapies represent the most common form of pharmacotherapy currently in use65 and have been shown to increase smoking cessation by 50% to 70%.66 In addition, studies are currently in progress to investigate smoking cessation after administration of nicotine vaccines (e.g., NicVAX and NIC002), which reduce the amount of nicotine that reaches the brain. First- and second-line pharmacologic treatments now provide additional strategies for smoking cessation. These alternative agents include antidepressants, such as the norepinephrine and dopamine reuptake inhibitors bupropion (e.g., Wellbutrin and Zyban) and nortriptyline (e.g., Aventyl and Pamelor), as well as the second-line pharmacotherapy alpha-agonist hypotensive agent clonidine (e.g., Catapres, Dixarit, and Kapvay).67 In 1997, bupropion hydrochloride (Zyban), an antidepressant not associated with sexual dysfunction,68–75 was the first nonnicotine pharmacotherapy to be approved by the FDA as a smoking cessation drug.65 Many studies have since reported on the effectiveness (doubled the odds of smoking cessation versus placebo) and safety of bupropion.76,77 However, the nicotine receptor partial agonist varenicline (Chantix) has been shown to be more effective for smoking cessation than both NRTs and nicotine agonists78,79 and three times more effective than placebo when administered with an established fixed quit date.82–82 In a phase III clinical trial, treatment with varenicline tartrate (1 mg twice a day) resulted in 43.9% participant abstinence (n = 344) compared with 29.8% abstinence (n = 342) with bupropion (Zyban, 150 mg twice a day) and 17.5% abstinence with placebo (n = 341).78 This increased efficacy with varenicline has been demonstrated in other clinical trials as well, such as the study by Rennard et al.82 Varenicline is potentially the most effective smoking cessation pharmacotherapy to date. As a result, studies have been and continue to be conducted by Gritz and others to maximize the positive translation of this benefit through physician- and pharmacist-based training and counseling into routine practice.84–84 In 2008, an updated guideline report was issued by the U.S. Public Health Service providing specific recommendations for brief and intensive tobacco cessation clinical interventions, as well as system-level changes designed to promote the assessment and treatment of tobacco dependence.85 Potential use of the carotenoid beta-carotene as an effective chemopreventive strategy for lung cancer was investigated in two randomized trials, the Alpha-Tocopherol, Beta-Carotene Cancer (ATBC) trial and CARET. Unfortunately, results of these studies showed that beta-carotene significantly increased risk of lung cancer in current smokers rather than decreasing it.86,87 In a secondary prevention setting of 1166 patients with stage I non–small cell lung cancer (SCLC), the Lung Intergroup Trial demonstrated no reduction in rates of SPTs, recurrences, or mortality in persons treated with isotretinoin and further identified, in both secondary subset analyses and a 6.2-year follow-up, increased overall and cancer-related mortality in current smokers receiving isotretinoin.88,89 Clark and colleagues90 conducted a phase III selenium skin cancer prevention study with secondary end points of other cancers. Although they identified no reduction in skin cancer associated with selenium treatment, secondary analyses suggested a decrease in both prostate and lung cancer. This information led to the development of the Selenium and Vitamin E Cancer Prevention Trial (SELECT) for the prevention of prostate cancer. A secondary end point was incidence of lung cancer.91 Contrary to the findings by Clark et al., the results of this study identified no decrease in prostate or lung cancer associated with selenium treatment.92 Another phase III trial testing selenium for lung cancer prevention was conducted through the cooperative groups. Results from this trial did not identify a reduction in lung cancer recurrence or in second primary lung cancer associated with selenium supplements.93 Many drugs have been shown to prevent lung cancer in preclinical studies.94 To overcome the difficulties associated with inhalation of tobacco smoke in animal models, Witschi95 has described a reproducible tobacco smoke carcinogenesis model using strain A/J mice, which develop lung tumors after exposure to suspended tobacco smoke particulates, and identifies the preventive potential of a mixture of myo-inositol and dexamethasone. Among the agents that have been tested in animal models are indole-3-carbinol,98–98 Kava,99 8-methyoxypsoralen,102–102 myo-inositol,103–106 PEITC,107–113 Polyphenon E,114–122 rapamycin,121 and silibinin.125–125 After preclinical studies, a phase I clinical study was conducted to establish the chemopreventive potential, maximum tolerated dose, and toxicity of myo-inositol in smokers with bronchial dysplasia.126 Results from this dose escalation (12-30 g/day) study showed that 3-month treatment with myo-inositol increased regression of preexisting dysplastic lesions by more than 40% compared with placebo. In addition, myo-inositol (18 g/day) was found to be safe and well tolerated. Veronesi et al.127 layered a phase IIb clinical trial of inhaled budesonide within a phase III LD-CT screening trial currently underway at the European Institute of Oncology. Participants of the phase II chemoprevention trial included 202 of the 5203 current and former smokers who had been recruited for the phase III screening trial. Results recently published demonstrated that budesonide had no significant effect on reduction of lung nodule size compared with placebo (2% versus 1% effect, respectively). However, both nonsolid and partially solid nodules (the types most likely to represent adenocarcinoma precursors) exhibited reduction in size, suggesting the potential for effective treatment within this defined patient population. Another promising chemopreventive agent for lung cancer is iloprost, a prostaglandin analogue often used to treat current and former smokers experiencing pulmonary hypertension. Results reported recently from a 6-year multicenter phase II clinical trial conducted by Keith et al.128 demonstrated significant improvement of endobronchial histology in former smokers treated with iloprost. Treatment was dose-escalated from 50 mg/day to 150 mg/day iloprost clathrate within the first 2 months, then maintained at 150 mg/day for the duration of treatment. Participants underwent two bronchoscopies, one before randomization and one after the end of treatment. The primary end point of the study was the difference between before treatment and after treatment bronchoscopy average histologic scores. Keith et al.128 identified significant histologic improvement in former smokers (58.6%) compared with placebo (28.6%), and treatment was reasonably well tolerated. This improvement was not observed within current smokers, suggesting a need to conduct individual studies for these two patient populations. However, because pulmonary hypertension is common among patients with chronic obstructive pulmonary disease, treatment of persons who have chronic obstructive pulmonary disease with iloprost could have a twofold benefit by decreasing the degree of pulmonary hypertension and reducing the risk of lung cancer. Based on the results of this study, a large phase III clinical trial is currently in development. Other potential preventive agents for lung cancer currently supported for use by the Division of Cancer Prevention at the NCI include epidermal growth factor receptor (EGFR)–tyrosine kinase inhibitors (e.g., erlotinib and gefitinib), polyphenols (e.g., green tea catechins and grape seed polyphenols), retinoids (e.g., all-trans-retinoic acid tretinoin, 9-cis-retinoic acid alitretinoin, 13-cis-retinoic acid isotretinoin, 4-hydroxy(phenyl)retinamide, and (4-HPR)-fenretinide), prostacyclin agonists, selenium compounds (e.g., selenized yeast, selenomethionine, and selenomethylsysteine), and antioxidants containing sulfur (e.g., anethole trithione).129 In addition, the NCI is currently funding a phase II biomarker prevention study targeting the effects of phenethyl isothiocyanate in the prevention of SCLC and non-SCLC.130 Lung carcinogenesis can encompass 20 to 30 years of a person’s life.131 Therefore effective strategies focused on prevention rather than on the treatment of established lung cancer have become the focus of many studies. Primary prevention methods, such as smoking avoidance and cessation, in combination with effective chemoprevention, particularly within high-risk populations, could provide the means to significantly decrease lung cancer development in the future.131 Screening trials for lung cancer were initiated in the 1970s and were based on chest radiography and sputum cytology. However, given the lack of evidence that current screening methods, including chest radiographs and sputum cytology, are associated with any significant decrease in lung cancer mortality, an effective strategy for lung screening has yet to be identified.132,133 Furthermore, these methods produce a significant number of false-positive tests, resulting in unnecessary invasive diagnostic treatments and procedures. With improved prognosis directly dependent on early detection, new strategies targeting early detection of lung cancer remain critical. Other potential screening methods are therefore being developed, including LD-CT scans, which have recently been reported to be associated with improved survival rates compared with standard chest radiographs.40 Likewise, positron emission tomography scans provide an alternative method of early detection, although they are expensive and are associated with significant radiation exposure. Ultimately, future strategies targeting the prevention of lung cancer must emphasize primary prevention, secondary prevention, and prevention of SPTs. A summary of lung cancer screening trials is provided in Table 23-7. Table 23-7 Select Lung Cancer Screening Studies The Prostate, Lung, Colorectal and Ovarian133 cancer screening trial enrolled 154,901 men and women (55 to 74 years of age) at 10 screening centers within the United States from 1993 to 2001.134 With regard to lung cancer, participants received either annual screening with a chest radiograph (n = 77,445) or usual medical care (n = 77,456) and were followed up for lung cancer mortality as a primary end point. In 2011, the 13-year follow-up report identified no reduction in lung cancer mortality associated with annual chest radiograph screening compared with usual care.133 The National Lung Screening Trial compared the effectiveness of LD-CT versus standard chest radiography in the early detection of lung cancer, also with a primary end point of lung cancer mortality. From 2002 to 2004, 53,454 participants were accrued from 33 centers across the United States.40 Participant recruitment was limited to high-risk patients (persons 50 to 74 years of age with extensive smoking histories [30 pack-years or more]) who quit smoking within the last 15 years and have received LD-CT. Three screenings were conducted, one at baseline and two at annual follow-up examinations, and quality of life and cost-effectiveness data were collected; study follow-up extended through 2009. Recently published results identified a 20% reduction in lung cancer mortality in high-risk patients, who also showed a 7% lower overall mortality rate.40 These findings arguably represent the most significant and impactful result in lung cancer management to date, other than tobacco prevention and cessation. However, the percentage of false-positive screens identified in the study was relatively high in comparison with other screening analyses.135 Specifically, of the 75,126 total CT screens, 18,146 (24.2%) tested positively, whereas only 649 (0.9%) led to diagnoses of lung cancer, representing a false-positive screening rate of 23.3%.40 The percentage of false-positive results was lower but remained significant for chest radiograph screening with a 6.5% false-positive rate. The Detection and Screening of Early Lung Cancer trial is also currently underway in Italy.136
Cancer Prevention, Screening, and Early Detection
Introduction
Carcinogenesis
Risk Modeling
Prevention
General Prevention/Lifestyle Intervention
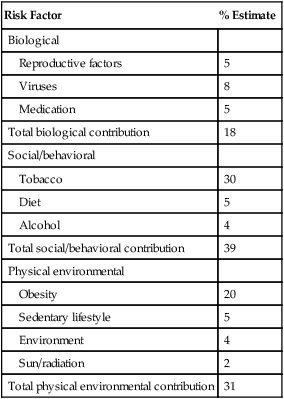
Risk Factor
% Estimate
Biological
Reproductive factors
5
Viruses
8
Medication
5
Total biological contribution
18
Social/behavioral
Tobacco
30
Diet
5
Alcohol
4
Total social/behavioral contribution
39
Physical environmental
Obesity
20
Sedentary lifestyle
5
Environment
4
Sun/radiation
2
Total physical environmental contribution
31
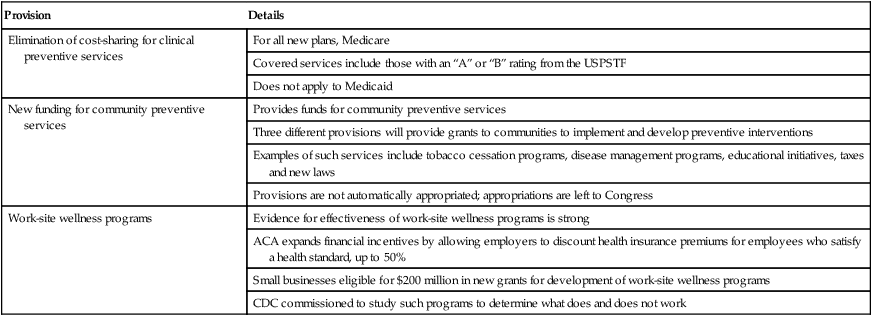
Provision
Details
Elimination of cost-sharing for clinical preventive services
For all new plans, Medicare
Covered services include those with an “A” or “B” rating from the USPSTF
Does not apply to Medicaid
New funding for community preventive services
Provides funds for community preventive services
Three different provisions will provide grants to communities to implement and develop preventive interventions
Examples of such services include tobacco cessation programs, disease management programs, educational initiatives, taxes and new laws
Provisions are not automatically appropriated; appropriations are left to Congress
Work-site wellness programs
Evidence for effectiveness of work-site wellness programs is strong
ACA expands financial incentives by allowing employers to discount health insurance premiums for employees who satisfy a health standard, up to 50%
Small businesses eligible for $200 million in new grants for development of work-site wellness programs
CDC commissioned to study such programs to determine what does and does not work
Infectious Agent
Associated Cancer
Intervention
Hepatitis B virus
Hepatocellular carcinoma
Hepatitis B vaccine, interferon therapy, nucleoside analogues
Hepatitis C virus
Hepatocellular carcinoma
Interferon therapy, nucleoside analogues
Human immunodeficiency virus
Kaposi sarcoma and non-Hodgkin lymphoma
Antiretroviral therapies
Helicobacter pylori
Gastric/stomach cancer
Antibiotics—“triple therapy”
Schistosomiasis
Bladder cancer
Antischistosomal agents—Praziquantel and Metrifonate
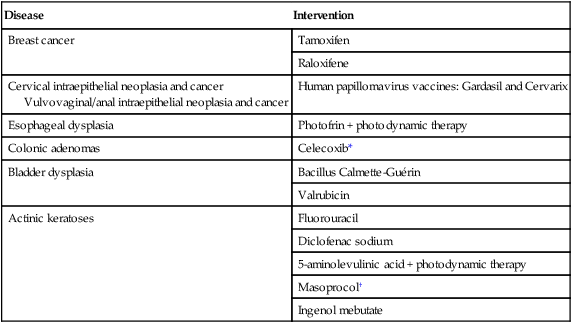
Chemoprevention
Disease
Intervention
Breast cancer
Tamoxifen
Raloxifene
Cervical intraepithelial neoplasia and cancer
Vulvovaginal/anal intraepithelial neoplasia and cancer
Human papillomavirus vaccines: Gardasil and Cervarix
Esophageal dysplasia
Photofrin + photodynamic therapy
Colonic adenomas
Celecoxib*
Bladder dysplasia
Bacillus Calmette-Guérin
Valrubicin
Actinic keratoses
Fluorouracil
Diclofenac sodium
5-aminolevulinic acid + photodynamic therapy
Masoprocol†
Ingenol mebutate
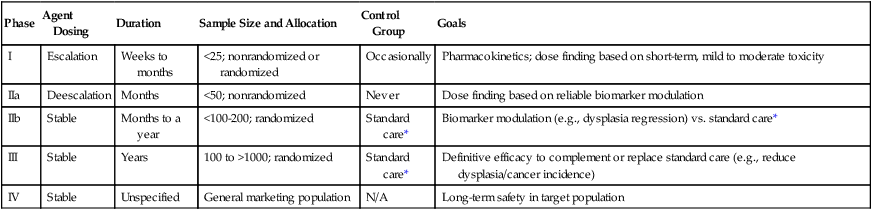
Phase
Agent Dosing
Duration
Sample Size and Allocation
Control Group
Goals
I
Escalation
Weeks to months
<25; nonrandomized or randomized
Occasionally
Pharmacokinetics; dose finding based on short-term, mild to moderate toxicity
IIa
Deescalation
Months
<50; nonrandomized
Never
Dose finding based on reliable biomarker modulation
IIb
Stable
Months to a year
<100-200; randomized
Standard care*
Biomarker modulation (e.g., dysplasia regression) vs. standard care*
III
Stable
Years
100 to >1000; randomized
Standard care*
Definitive efficacy to complement or replace standard care (e.g., reduce dysplasia/cancer incidence)
IV
Stable
Unspecified
General marketing population
N/A
Long-term safety in target population
Screening and Early Detection
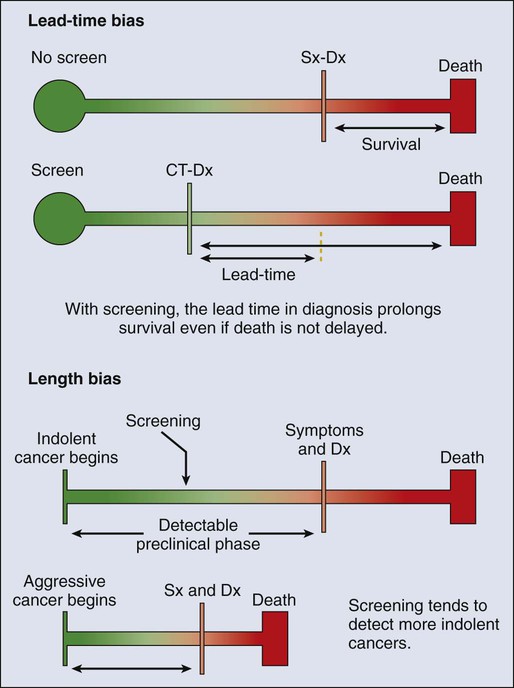
Certainty of Net Benefit
Magnitude of Net Benefit
Substantial
Moderate
Small
Zero/Negative
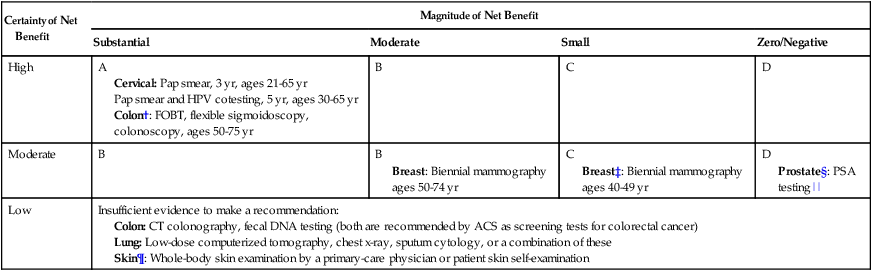
High
A
Cervical: Pap smear, 3 yr, ages 21-65 yr
Pap smear and HPV cotesting, 5 yr, ages 30-65 yr
Colon†: FOBT, flexible sigmoidoscopy, colonoscopy, ages 50-75 yr
B
C
D
Moderate
B
B
Breast: Biennial mammography ages 50-74 yr
C
Breast‡: Biennial mammography ages 40-49 yr
D
Prostate§: PSA testing||
Low
Insufficient evidence to make a recommendation:
Lung Cancer
Risk Factors/Etiology
Risk Modeling/Assessment
Prevention
Chemoprevention
Vitamins and Minerals
Other Novel Agents
Screening
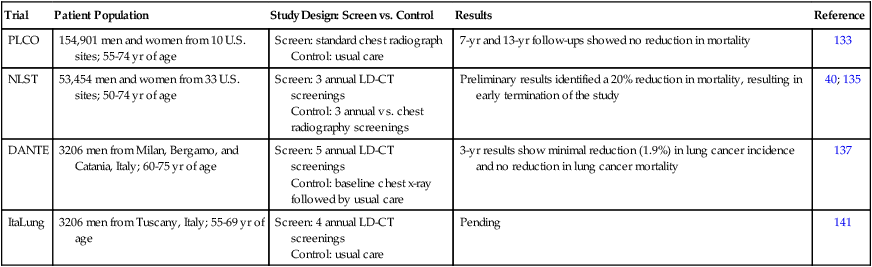
Trial
Patient Population
Study Design: Screen vs. Control
Results
Reference
PLCO
154,901 men and women from 10 U.S. sites; 55-74 yr of age
Screen: standard chest radiograph
Control: usual care
7-yr and 13-yr follow-ups showed no reduction in mortality
133
NLST
53,454 men and women from 33 U.S. sites; 50-74 yr of age
Screen: 3 annual LD-CT screenings
Control: 3 annual vs. chest radiography screenings
Preliminary results identified a 20% reduction in mortality, resulting in early termination of the study
40; 135
DANTE
3206 men from Milan, Bergamo, and Catania, Italy; 60-75 yr of age
Screen: 5 annual LD-CT screenings
Control: baseline chest x-ray followed by usual care
3-yr results show minimal reduction (1.9%) in lung cancer incidence and no reduction in lung cancer mortality
137
ItaLung
3206 men from Tuscany, Italy; 55-69 yr of age
Screen: 4 annual LD-CT screenings
Control: usual care
Pending
141
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Cancer Prevention, Screening, and Early Detection