Cancer Pain: Principles of Assessment and Syndromes
Nathan I. Cherny
Surveys indicate that pain is experienced by 30–60% of patients with cancer during active therapy and by more than two thirds of those with advanced disease (1). Unrelieved pain is incapacitating and precludes a satisfying quality of life; it interferes with physical functioning and social interaction and is strongly associated with heightened psychological distress. It can provoke or exacerbate existential distress, (2) disturb normal processes of coping and adjustment, and augment a sense of vulnerability, contributing to a preoccupation with the potential for catastrophic outcomes. Persistent pain interferes with the ability to eat, sleep, think, and interact with others and is correlated with fatigue in patients with cancer.
The relationship between pain and psychological well-being is complex and reciprocal; mood disturbance and beliefs about the meaning of pain in relation to illness can exacerbate perceived pain intensity, and the presence of pain is a major determinant of function and mood (3).
The high prevalence of chronic pain among patients with cancer, and the profound psychological and physical burdens engendered by this symptom, oblige all treating clinicians to be skilled in pain management (4). Providing relief from pain in patients with cancer is an ethical imperative, and it is incumbent upon clinicians to maximize the knowledge, skill, and diligence needed to attend to this task.
The undertreatment of cancer pain has many causes, among the most important of which is inadequate assessment (5, 6). In a study to evaluate the correlation between patient and clinician evaluation of pain severity, Grossman et al. (6) found that when patients rated their pain as moderate to severe, oncology fellows failed to appreciate the severity of the problem in 73% of cases. In studies of pain relief among patients with cancer in the United States (7) and France (8), the discrepancy between patient and physician evaluation of the severity of the pain problem was a major predictor of inadequate relief.
Cancer Pain Syndromes
A woman with breast cancer who presents with shoulder pain may have any one of a number of pain syndromes including postoperative frozen shoulder, taxol- or bisphosphonate-associated proximal myalgias, radiation or malignant upper brachial plexopathy, metastases in the bony structures of the shoulder, impending fracture of the proximal humerus, C4 radiculopathy associated with epidural encroachment or leptomeningeal metastases, hepatic capsular distension, or a benign pathology unrelated to the cancer. To arrive at an appropriate therapeutic plan, the treating clinician must be aware of the range of possible causes of the pain, their distinguishing clinical features, and efficient diagnostic strategies to isolate the specific cause as quickly and easily as possible. Lack of awareness of the range of diagnostic possibilities may result in undertreatment.
Cancer pain syndromes are defined by the association of particular pain characteristics and physical signs with specific consequences of the underlying disease or its treatment. Syndromes are associated with distinct etiologies and pathophysiologies and have important prognostic and therapeutic implications. Pain syndromes associated with cancer can be either acute or chronic. Whereas acute pains experienced by patients with cancer are usually related to diagnostic and therapeutic interventions (Table 1.1), chronic pains are most commonly caused by direct tumor infiltration. Adverse consequences of cancer therapy, including surgery, chemotherapy, and radiation therapy, account for 15–25% of chronic cancer pain problems, and a small proportion of the chronic pains experienced by patients with cancer is caused by pathology unrelated to either the cancer or the cancer therapy.
Chronic Pain Syndromes
Most chronic cancer-related pains are caused directly by the tumor (Table 1.2). Data from the largest prospective survey of cancer pain syndromes revealed that almost one fourth of the patients experienced two or more pains. Over 90% of the patients had one or more tumor-related pains and 21% had one pain or more caused by cancer therapies. Somatic pains (71%) were more common than neuropathic (39%) or visceral (34%) pains (9). Bone pain and compression of neural structures are the two most common causes of chronic pain (10, 11, 12, 13, 14).
Bone Pain
Bone metastases are the most common cause of chronic pain in patients with cancer. Cancers of the lung, breast, and prostate most often metastasize to bone, but any tumor type may be complicated by painful bony lesions. Although bone pain is usually associated with direct tumor invasion of bony structures, > 25% of patients with bony metastases are pain free (15), and patients with multiple bony metastases
typically report pain in only a few sites. The factors that convert a painless lesion to a painful one are unknown.
typically report pain in only a few sites. The factors that convert a painless lesion to a painful one are unknown.
Table 1.1 Cancer-Related Acute Pain Syndromes | |
|---|---|
|
Pathophysiology
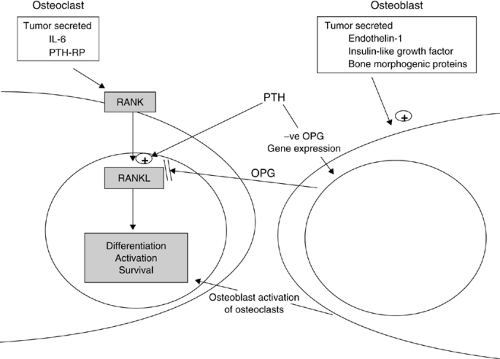
Bone metastases could potentially cause pain by any of multiple mechanisms, including endosteal or periosteal nociceptor activation (by mechanical distortion or release of chemical mediators) or tumor growth into adjacent soft tissues and nerves (16). There have been profound changes in the understanding of the physiology of bone pain. As cancer grows in bone, a wide range of inflammatory mediators is released. The cytokine expression of the tumor cells and their interaction with osteoclasts and osteoblasts determine the nature of the lesion (Fig. 1.1)). Osteoclast activity is regulated by a complex set of interactions. Activation of the osteoclast receptor nuclear factor-κB (RANK) leads to the downstream activation of nuclear factor-κB receptor ligand (RANKL), which leads to differentiation, activation, and survival of osteoclasts. Opposing this, the osteoblast-expressed protein osteoprotegerin (OPG), neutralizes RANKL and prevents the activation of RANK (17). Parathyroid Hormone (PTH) is involved in this interaction by upregulating RANKL and by inhibiting OPG gene expression. Recently it has been found that myeloma cells directly express RANKL and indicate that specific blockade of RANKL may be an effective treatment for myeloma bone disease (18). Osteoclastic activity has been shown to be increased by the expression of interleukin-6 and parathyroid hormone–related protein by the tumor (19).
Osteoblastic metastases can be caused by tumor-secreted endothelin-1 (ET-1), insulin-like growth factor, and a variety of other potential osteoblastic factors (20). Among these are the osteogenic factors released by the prostate and other cancer types, called bone morphogenic proteins (BMPs), which are part of the transforming growth factor (TGF)-β family (21, 22, 23). Paradoxically, the stimulation of osteoblasts can increase osteoclast function because osteoblasts are the main regulators of bone-destroying osteoclasts. Therefore, the expression of osteolytic and osteoblastic factors can produce mixed metastases or increased osteolysis.
Table 1.2 Cancer-Related Chronic Pain Syndromes | |
|---|---|
|
The presence of bone metastases stimulates an upregulation of peripheral and central neural processes. Immunocytochemical studies from mouse models indicate alterations in the dorsal horn, including astrocyte hypertrophy and upregulation of dynorphin, that are quite different from those seen in inflammatory and neuropathy pains. In addition, in vivo electrophysiology of individual dorsal horn neurons have indicated a profound change and increased excitation within superficial and deep laminae (I and V, respectively). Additionally, there is an increase in the proportion of wide dynamic range (WDR) nerves in lamina I; this is correlated with an increased response to electrical and mechanical stimuli and parallels the development of hyperalgesia and allodynia (24).
Differential Diagnosis
Bone pain due to metastatic tumor needs to be differentiated from less common causes, among which the nonneoplastic causes include osteoporotic fractures (including those associated with multiple myeloma); focal osteonecrosis, which may be idiopathic or related to chemotherapy, corticosteroids (25), or radiotherapy (see subsequent text); and osteomalacia (26).
Multifocal or Generalized Bone Pain
Bone pain may be focal, multifocal, or generalized. Multifocal bone pains are most commonly experienced by patients with
multiple bony metastases. A generalized pain syndrome is also rarely produced by replacement of bone marrow (27, 28, 29, 30). This bone marrow replacement syndrome has been observed in hematogenous malignancies (31, 32, 33) and, less commonly, in solid tumors (28). This syndrome can occur in the absence of abnormalities on bone scintigraphy or radiography, increasing the difficulty of diagnosis. Rarely, a paraneoplastic osteomalacia, which is associated with elevated levels of fibroblast growth factor 23 (34), can mimic multiple metastases (35).
multiple bony metastases. A generalized pain syndrome is also rarely produced by replacement of bone marrow (27, 28, 29, 30). This bone marrow replacement syndrome has been observed in hematogenous malignancies (31, 32, 33) and, less commonly, in solid tumors (28). This syndrome can occur in the absence of abnormalities on bone scintigraphy or radiography, increasing the difficulty of diagnosis. Rarely, a paraneoplastic osteomalacia, which is associated with elevated levels of fibroblast growth factor 23 (34), can mimic multiple metastases (35).
Vertebral Syndromes
The vertebrae are the most common sites of bony metastases. More than two thirds of vertebral metastases are located in the thoracic spine; lumbosacral and cervical metastases account for approximately 20% and 10%, respectively (36, 37). Multiple-level involvement is common, occurring in >85% of patients (38). The early recognition of pain syndromes due to tumor invasion of vertebral bodies is essential because pain usually precedes the compression of adjacent neural structures, and prompt treatment of the lesion may prevent the subsequent development of neurologic deficits. Several factors often confound accurate diagnosis; referral of pain is common, and the associated symptoms and signs can mimic a variety of other disorders, both malignant (e.g., paraspinal masses) and nonmalignant.
Atlantoaxial destruction and odontoid fracture
Nuchal or occipital pain is the typical presentation of destruction of the atlas or fracture of the odontoid process. Pain often radiates over the posterior aspect of the skull to the vertex and is exacerbated by movement of the neck, particularly flexion (39). Pathologic fracture may result in secondary subluxation with compression of the spinal cord at the cervicomedullary junction. This complication is usually insidious and may begin with symptoms or signs in one or more extremity. Typically, there is early involvement of the upper extremities and the occasional appearance of so-called pseudo-levels suggestive of more caudal spinal lesions; these deficits can slowly progress to involve the sensory, motor, and autonomic functions (40). Magnetic resonance imaging (MRI) is probably the best method for visualizing this region of the spine (39), but clinical experience suggests that computed tomography (CT) is also sensitive. Plain radiography, tomography, and bone scintigraphy should be viewed as ancillary procedures.
C7-T1 syndrome
Invasion of the C7 or T1 vertebra can result in pain referred to the interscapular region. These lesions may be missed if radiographic evaluation is mistakenly targeted to the painful area caudal to the site of damage. Additionally, visualization of the appropriate region on routine radiographs may be inadequate because of obscuration by overlying bone and mediastinal shadows. Patients with interscapular pain should therefore undergo radiography of both the cervical and the thoracic spine. Bone scintigraphy may assist in targeting additional diagnostic imaging procedures such as CT or MRI that can be useful in assessing the possibility of pain being referred from an extraspinal site, such as the paraspinal gutter.
T12-L1 syndrome
A T12 or L1 vertebral lesion can refer pain to the ipsilateral iliac crest or the sacroiliac joint. Imaging procedures directed at pelvic bones can miss the source of the pain.
Sacral syndrome
Severe focal pain radiating to buttocks, perineum, or posterior thighs may accompany destruction of
the sacrum (41). The pain is often exacerbated by sitting or lying down and is relieved by standing or walking (42). The neoplasm can spread laterally to involve muscles that rotate the hip (e.g., the pyriformis muscle). This may produce severe incident pain induced by motion of the hip or a malignant “pyriformis syndrome,” characterized by buttock or posterior leg pain that is exacerbated by internal rotation of the hip. Local extension of the tumor mass may also involve the sacral plexus (see subsequent text).
the sacrum (41). The pain is often exacerbated by sitting or lying down and is relieved by standing or walking (42). The neoplasm can spread laterally to involve muscles that rotate the hip (e.g., the pyriformis muscle). This may produce severe incident pain induced by motion of the hip or a malignant “pyriformis syndrome,” characterized by buttock or posterior leg pain that is exacerbated by internal rotation of the hip. Local extension of the tumor mass may also involve the sacral plexus (see subsequent text).
Imaging Investigations of Bone Pain
The two most important imaging modalities for the evaluation of bone pain are plain radiography and nuclear bone scan. In general, CT and MRI are reserved for situations in which the diagnosis cannot be discerned from clinical information and baseline tests in which there are specific diagnostic issues to be resolved that require special techniques.
Plain radiography
Radiography should be the first test ordered to evaluate bone pain and confirm findings of other imaging studies. There are three radiographic patterns of metastatic disease: osteolytic, osteoblastic, and mixed. Osteoblastic areas correspond to the reaction of the host bone to the metastases. This reactive bone forms in a random pattern lacking normal bone structure and often lacking mechanical strength despite its sclerotic, radio-opaque appearance. Lytic lesions with little or no reactive bone formation indicate bone destruction in excess of bone formation. Periosteal thickening or elevation is commonly seen with primary bone neoplasms, rapidly growing tumors, or stress fractures through the underlying bone. When examining bone radiographs of long bones it is important to evaluate the extent of cortical destruction. The risk of pathologic fracture is high if 50% or more of the cortex is destroyed by the tumor (43, 44, 45). Vertebral bodies must be carefully examined for collapse (best viewed on a lateral radiograph) and pedicle erosion (viewed on an anterior/posterior radiograph) because both these findings are associated with enhanced risk of epidural encroachment by tumor.
Bone scan
Technetium bisphosphonate bone scans are valuable in evaluating patients with multifocal pain and in identifying the extent of bony secondaries (46). Three patterns of uptake may indicate bony metastases. The radioisotope most commonly accumulates in the reactive new bone, giving rise to a “hot spot.” Less frequently, metastases give rise to cold spots because of the complete absence of reactive bone or poor blood flow (47) or to a pattern of diffuse accumulation of tracer throughout the skeleton (superscan) in the setting of disseminated skeletal disease.
There are several problems associated with bone scans:
Bone scans are characterized by high sensitivity and low specificity. Uptake may occur at any skeletal site with an elevated rate of bone turnover in conditions such as trauma (even remote trauma), infection, arthropathy, or even acute osteopenia caused by disuse (46, 48, 49, 50). Whereas a scan showing multiple lesions strongly suggests metastases, only 50% of solitary foci represents metastases, and in such cases, radiographic correlation is essential.
Because bone scans do not evaluate the structural integrity of the bone, positive findings that correspond to painful sites should be further evaluated by plain radiographs, CT scan, or both.
Other radionucleotide bone scanning techniques are occasionally used. Single photon emission computed tomography (SPECT) scanning is a bone scanning technique with improved sensitivity and specificity compared to conventional bone scanning (46, 54) and conventional positron emission tomography (PET) scanning techniques (55). In patients with diffuse tracer uptake, bone marrow scanning using a tracer linked to antigranulocyte antibody can be helpful in distinguishing a normal scan from a superscan caused by diffuse marrow infiltration (56). Gallium scanning is useful for detecting otherwise undetected bone metastases from lymphomas and soft tissue sarcomas (57).
Computed tomography
CT is a second-tier investigation technique used in the evaluation of bony secondaries. It is effective in evaluating the three-dimensional integrity of bone and to better visualize abnormal lesions identified on bone scan (58). It may be useful in confirming suspicions raised by bone scans and more clearly illustrating the extent of bone destruction. It is particularly helpful in the evaluation of patients with pain in the regions of the pelvic and shoulder girdles and base of the skull who have equivocal or nondiagnostic findings on plain radiography. Spine lesions can also be well visualized by CT using contemporary CT equipment and techniques. The additional yield from MRI is usually very limited.
When confirmation of histologic diagnosis is required, CT-guided biopsy or fine needle aspiration is usually diagnostic (59, 60, 61). When the lesion is osteolytic, CT-guided needle biopsy is usually satisfactory (diagnostic accuracy: 80%). When the lesion is osteoblastic or when there is a thick overlying cortical rim, it is extremely difficult to insert a needle and obtain an adequate tissue sample, and such cases may necessitate open surgical biopsy.
Magnetic resonance imaging
MRI is generally reserved for three clinical situations: to arbitrate suspicious lesions that remain ill defined despite plain radiography and CT scan, when bone marrow infiltration is suspected, and in the evaluation of spinal cord compression.
MRI is an excellent method to evaluate bone marrow involvement in diseases such as leukemia, lymphoma, and multiple myeloma that replace the marrow space. Because bone marrow (including hematopoietic or “red” marrow) contains a high percentage of fat, T1-weighted MRI scans generally reveal metastases as focal areas of low signal intensity (62). This approach has also been shown to be very sensitive to solid tumors that metastasize to bone marrow such as breast and lung cancer (63, 64, 65).
It is often difficult to distinguish between changes caused by treatment, fracture, and tumor. Indeed, noncontrast MRI cannot reliably distinguish between these changes. In one study the false-positive tumor detection rate was as high as 50% (66). Gadolinium-enhanced bone imaging can be helpful in this situation; tumors commonly demonstrate high or inhomogeneous signal intensity after gadolinium injection, which is not seen in fractures or postoperative changes (67).
Positron emission tomography ± computed tomography
18F-Fluoride PET/CT scan has higher sensitivity and specificity than PET scanning alone in detecting bone metastases. This may be a helpful approach in situations in which either CT scan or technetium Tc 99m-methylene diphosphonate bone scintigraphy is inconclusive. In a study, among the 12 patients with bone pain referred for 18F-fluoride PET/CT scan despite negative findings on the Tc 99m-methylene diphosphonate bone scintigraphy, the scan suggested malignant bone
involvement in all 4 patients with proved skeletal metastases, a potential benign cause in 4 of 7 patients who had no evidence of metastatic disease, and a soft tissue tumor mass invading a sacral foramen in 1 patient (70).
involvement in all 4 patients with proved skeletal metastases, a potential benign cause in 4 of 7 patients who had no evidence of metastatic disease, and a soft tissue tumor mass invading a sacral foramen in 1 patient (70).
Back Pain and Epidural Compression
Epidural compression (EC) of the spinal cord or cauda equina is the second most common neurologic complication of cancer, occurring in up to 10% of patients (71). In a large retrospective series, 0.23% of patients with cancer had EC at the presentation of the disease and 2.5% of patients dying of cancer had at least one admission for malignant spinal cord compression (MSCC) in the 5 years preceding their death (72). Breast, lung, and prostate cancers each account for 20–25% of the epidural compression events occurring as complications (72, 73). EC is mostly caused by posterior extension of the vertebral body metastasis to the epidural space. Occasionally, EC is caused by tumor extension from the posterior arch of the vertebra or by infiltration of a paravertebral tumor through the intervertebral foramen.
Untreated, EC inevitably leads to neurologic damage. Effective treatment can potentially prevent these complications. The most important determinant of the efficacy of treatment is the degree of neurologic impairment at the time therapy is initiated. Seventy-five percent of patients who begin treatment while they are ambulatory remain so; the efficacy of treatment declines to 30–50% for those who begin treatment while they are markedly paretic and is 10–20% for those who are plegic (74). Despite this, delays in diagnosis are commonplace (75).
Back pain is the initial symptom in almost all patients with EC (71, 76), and in 10% it is the only symptom at the time of diagnosis (77). Because pain usually precedes neurologic signs by a prolonged period, it should be viewed as a potential indicator of EC, which can lead to provision of treatment at a time when a favorable response is most likely. Back pain, however, is a nonspecific symptom that can result from bony or paraspinal metastases without epidural encroachment, retroperitoneal or leptomeningeal tumor, epidural lipomatosis due to steroid administration (78), or a large variety of other benign conditions. Because it is infeasible to pursue an extensive evaluation in every patient with cancer who develops back pain, the complaint should impel an evaluation that determines the likelihood of EC and thereby selects patients appropriate for definitive imaging of the epidural space. The selection process is based on symptoms and signs and the results of simple imaging techniques.
Clinical features of epidural extension
Some pain characteristics are particularly suggestive of epidural extension (79). Rapid progression of back pain in a crescendo pattern is an ominous occurrence (80). Radicular pain, which can be constant or lancinating, has similar implications (79). It is usually unilateral in the cervical and lumbosacral regions and bilateral in the thorax, where it is often experienced as a tight, belt-like band across the chest or abdomen (79). The likelihood of EC is also greater when back or radicular pain is exacerbated by recumbency, cough, sneeze, or strain (81). Other types of referred pain are also suggestive of EC, including Lhermitte’s sign (82) and central pain due to spinal cord compression, which is usually perceived at some distance below the site of the compression and is typically a poorly localized, nondermatomal dysesthesia (71).
Weakness, sensory loss, autonomic dysfunction, and reflex abnormalities usually occur after a period of progressive pain (79). Weakness may begin segmentally if related to nerve root damage or as a multisegmental or pyramidal distribution if the cauda equina or spinal cord, respectively, is injured. The rate of progression of weakness is variable; in the absence of treatment, one third of patients will develop paralysis within 7 days of the onset of weakness (83). Patients whose weakness progresses slowly have a better prognosis for neurologic recovery with treatment than those whose weakness progresses rapidly (84, 85). Without effective treatment, sensory abnormalities, which may also begin segmentally, may ultimately evolve to a sensory level, with complete loss of all sensory modalities below the site of injury. The upper level of sensory findings may correspond to the location of the epidural tumor or be below it by many segments (79). Ataxia without pain is the initial presentation of EC in 1% of patients; this finding is presumably related to the early involvement of the spinocerebellar tracts (36). Bladder and bowel dysfunction occur late, except in patients with a conus medullaris lesion who may present with acute urinary retention and constipation without preceding motor or sensory symptoms (79).
Other features that may be evident on examination of patients with EC include scoliosis, asymmetrical wasting of paravertebral musculature, and a gibbus (palpable stoop in the spinous processes). Spinal tenderness to percussion, which may be severe, often accompanies the pain.
Imaging modalities
Definitive imaging of the epidural space confirms the existence of EC (and thereby indicates the necessity and urgency of treatment), defines the appropriate radiation portals, and determines the extent of epidural encroachment (which influences prognosis and may alter the therapeutic approach). The options for definitive imaging include MRI, myelography and CT-myelography, or spiral CT without myelographic contrast.
MRI is noninvasive and offers accurate imaging of the vertebrae and intraspinal and paravertebral structures. When available it is generally the preferred mode of evaluation (73). Whenever possible, total spine imaging should be performed because multiple-level involvement is common and other sites may be clinically occult. In a study of 65 patients with cord compression, 32 (49%) showed had multiple-level involvement and of these, 18 (66%) were clinically occult (86). MRI has multiple advantages: metastases can be distinguished from other pathologic processes involving the axial skeleton, epidural and intradural space, and spinal cord. This is particularly true for bacterial abscesses; leptomeningeal carcinomatosis; intradural extramedullary or, rarely, intramedullary metastases or primary tumors; and infectious or inflammatory myelitis.
MRI is relatively contraindicated in patients with severe claustrophobia and certain metallic implants and is absolutely contraindicated for patients with cardiac pacemakers or aneurysm clips. Several other groups who may not be suitable for MRI include very obese patients and those with severe kyphosis or scoliosis.
Previously, myelography was considered the standard examination for imaging the spinal cord (87). In contrast to MRI or CT scan, it is invasive and evaluation may be limited if there is a complete block to the flow of contrast, which precludes the demonstration of the extent of the compressing lesion. It has the advantages of facilitating simultaneous evaluation of the cerebrospinal fluid (CSF) for cytology when leptomeningeal metastases are part of the differential diagnosis.
Postmyelographic CT is a useful tool that provides additional information about the vertebral and paravertebral structures. It can usually define the extent of the cord compression (88) and may help distinguish cord compression caused by displaced bony fragments from soft tissue extension and in the identification of paraspinal tumors with extension through the intervertebral foramina (89).
In addition to immediate patient discomfort, myelography is often complicated by postprocedural side effects that include back pain, headache, vomiting, seizures, and adverse
neurobehavioral reactions. The risk of adverse effects is related to the gauge and type of needle used (90), the contrast medium (91), and the anatomy of the EC.
neurobehavioral reactions. The risk of adverse effects is related to the gauge and type of needle used (90), the contrast medium (91), and the anatomy of the EC.
Similar to MRI, CT scanning is noninvasive and provides excellent visualization of the vertebrae, vertebral structural integrity, paravertebral soft tissues, and vertebral foramina. The improved resolution observed with contemporary spiral techniques facilitate clear imaging of the spinal canal contents. Although no comparative data are yet available, in the author’s experience, CT scanning of regions identified by either plain radiography or bone scan usually provides excellent visualization of cortical integrity, the intervertebral foramina, and the canal contents. Bone and soft tissue windows are used in a complimentary manner; bone windows allow evaluation of bony integrity and, in particular, cortical breach, while soft tissue windows are used to evaluate the contents of the spinal canal. Using this approach the more expensive and less readily available MRI can be reserved for equivocal cases, when leptomeningeal metastases are suspected, or when total spinal imaging is required.
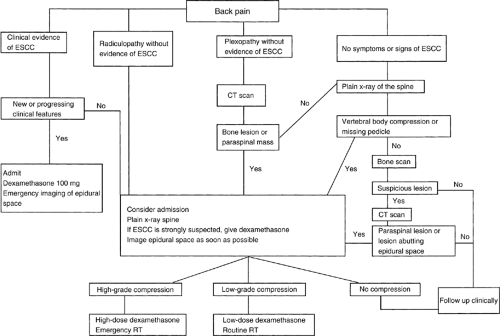
Algorithm for the investigation of cancer patients with back pain
Given the prevalence and the potentially dire consequences of EC, and the recognition that back pain is a marker of early (and therefore treatable) EC, algorithms have been developed to guide the evaluation of back pain in patients with cancer (Fig. 1.2)). The objective of these algorithms is to select a subgroup that should undergo definitive imaging of the epidural space from a large number of patients who develop back pain. Effective treatment of EC before irreversible neurologic compromise occurs is the overriding goal of these approaches.
Pain Syndromes of the Bony Pelvis and Hip
The pelvis and hip are common sites of metastatic involvement. Lesions may involve any of the three anatomic regions of the pelvis (i.e., ischiopubic, iliosacral, or periacetabular region), the hip joint itself, or the proximal femur (92). The weight-bearing function of these structures, which is essential for normal ambulation, contributes to the propensity of the disease at these sites to cause incident pain with ambulation.
Hip joint syndrome
Tumor involvement of the acetabulum or head of the femur typically produces localized hip pain that is aggravated by weight bearing and movement of the hip. The pain may radiate to the knee or medial thigh, and, occasionally, pain is limited to these structures (92, 93). Medial extension of an acetabular tumor can involve the lumbosacral plexus as it traverses the pelvic sidewall. Evaluation of this region is best accomplished with CT scan or MRI, both of which can demonstrate the extent of bony destruction and adjacent soft tissue involvement more sensitively than other imaging techniques (94). Important differential diagnoses include avascular necrosis, radicular pain (usually L1), or, occasionally, occult infections (95).
Acrometastases
Acrometastases, metastases in the hands and feet, are rare and often misdiagnosed or overlooked (96). In the feet, the larger bones containing the higher amount of red marrow, such as the os calcis, or talus are usually involved (97, 98). Symptoms may be vague and can mimic other conditions, such as osteomyelitis, gouty rheumatoid arthritis, Reiter’s syndrome, Paget’s disease, osteochondral lesions, and ligamentous sprains.
Arthritides
Hypertrophic Pulmonary Osteoarthropathy
Hypertrophic pulmonary osteoarthropathy (HPOA) is a paraneoplastic syndrome that incorporates clubbing of the fingers, periostitis of long bones, and, occasionally, a rheumatoid-like polyarthritis (99). Periosteitis and arthritis can produce pain, and tenderness and swelling in the knees, wrists, and ankles. The onset of symptoms is usually subacute and may precede the discovery of the underlying neoplasm by several months. It is most commonly associated with non–small cell lung cancer. Less commonly, it mat be associated with benign mesothelioma (100), pulmonary metastases from other sites (101), smooth muscle tumors of the esophagus (102), breast cancer (103), and metastatic nasopharyngeal cancer (104). Effective antitumor therapy is sometimes associated with symptom regression (105). HPOA is diagnosed on the basis of physical findings, radiologic appearance, and radionuclide bone scan (99, 106, 107).
Other Polyarthritides
Rarely, rheumatoid arthritis, systemic lupus erythematosus, and asymmetric polyarthritis may occur as paraneoplastic phenomena that resolve with effective treatment of the underlying disease (108). A syndrome of palmar plantar fasciitis and polyarthritis characterized by palmar and digital fibromatosis with polyarticular painful capsular contractions, has been associated with ovarian (109), breast (110) and gastric (111) cancers.
Muscle Pain
Muscle Cramps
Persistent muscle cramps in patients with cancer are usually caused by an identifiable neural, muscular, or biochemical abnormality (112). In one series of 50 patients, 22 had peripheral neuropathy, 17 had root or plexus pathology (including 6 with leptomeningeal metastases), 2 had polymyositis, and 1 had hypomagnesemia. In this series, muscle cramps were the presenting symptom of recognizable and previously unsuspected neurologic dysfunction in 64% (27 of 42) of the identified causes (113). Cramps have been reported as an adverse effect of imatinib (114), goserelin (115), and vincristine (116).
Skeletal Muscle Tumors
Soft tissue sarcomas arising from fat, fibrous tissue, or skeletal muscle are the most common tumors involving the skeletal muscles. The skeletal muscle is one of the most unusual sites of metastasis from any malignancy (117, 118). The sarcomas occur disproportionately at sites of prior muscle trauma (119). Lesions are usually painless but may present with persistent ache.
Headache and Facial Pain
Headache in the patient with cancer results from traction, inflammation, or infiltration of pain-sensitive structures in the head or neck. Early evaluation with appropriate imaging techniques may identify the lesion and allow prompt treatment, which may reduce pain and prevent the development of neurologic deficits (120).
Intracerebral Tumor
Among 183 patients with new-onset chronic headache as an isolated symptom, investigation revealed underlying intracerebral tumor in 15 cases (121). The prevalence of headache in patients with brain metastases or primary brain tumors is 60–90% (122, 123). The headache is presumably produced by traction on pain-sensitive vascular and dural tissues. Patients with multiple metastases and those with posterior fossa metastases are more likely to report this symptom (124). The pain may be focal, overlying the site of the lesion, or generalized. Headache has lateralizing value, especially in patients with supratentorial lesions (125). Posterior fossa lesions often cause a bifrontal headache. The quality of the headache is usually throbbing or steady, and the intensity is usually mild to moderate (125).
Among children, clinical features predictive of underlying tumor include sleep-related headache, headache in the absence of a family history of migraine, vomiting, absence of visual symptoms, headache of <6 months’ duration, confusion, and abnormal neurologic examination findings (126).
The headache is often worse in the morning and is exacerbated by stooping, sudden head movement, or Valsalva’s maneuvers (cough, sneeze, or strain) (125). In patients with increased intracranial pressure, these maneuvers can also precipitate transient elevations in intracranial pressure called plateau waves, which may also be spontaneous and can be associated with short periods of severe headache, nausea, vomiting, photophobia, lethargy, and transient neurologic deficits (127, 128). Occasionally, these plateau waves produce life-threatening herniation syndromes (127, 128).
Leptomeningeal Metastases
Leptomeningeal metastases, which are characterized by diffuse or multifocal involvement of the subarachnoid space by metastatic tumor, occur in 1–8% of patients with systemic cancer (129). Non-Hodgkin’s lymphoma and acute lymphocytic leukemia both demonstrate a predilection for meningeal metastases (129); the incidence is lower for solid tumors alone. Among solid tumors, adenocarcinomas of the breast and small cell lung cancer predominate (130).
Leptomeningeal metastases present with focal or multifocal neurologic symptoms or signs that may involve any level of the neuraxis (129, 131, 132). More than one third of patients present with evidence of cranial nerve damage, including double vision, hearing loss, facial numbness, and decreased vision (131, 132); this is particularly true among patients with underlying hematologic malignancy (132). Less common features include seizures, papilledema, hemiparesis, ataxic gait, and confusion (133). Generalized headache and radicular pain in the low back and buttocks are the most common pains associated with leptomeningeal metastases (132). The headache is variable and may be associated with changes in mental status (e.g., lethargy, confusion, or loss of memory), nausea, vomiting, tinnitus, or nuchal rigidity. Pains that resemble cluster headache (134) or glossopharyngeal neuralgia with syncope (135) have also been reported.
The diagnosis of leptomeningeal metastases is confirmed through analysis of the CSF, which may reveal elevated pressure, elevated protein level, depressed glucose level, and/or lymphocytic pleocytosis. Ninety percent of patients ultimately show positive cytology, but multiple evaluations may be required. After a single lumbar puncture (LP), the false-negative rate may be as high as 55%; this falls to only 10% after three LPs (131, 136, 137). The sensitivity and specificity of CSF cytology is enhanced by the use of fluorescence insitu hybridization (FISH) (138, 139) or immunocytochemical techniques (140). Tumor markers, such as lactic dehydrogenase (LDH) isoenzymes (131), carcinoembryonic antigen (141),
β2-microglobulin (141), and tissue polypeptide antigen (142), may help delineate the diagnosis. Flow cytometry for detection of abnormal deoxyribonucleic acid content may be a useful adjunct to cytologic examination (143).
β2-microglobulin (141), and tissue polypeptide antigen (142), may help delineate the diagnosis. Flow cytometry for detection of abnormal deoxyribonucleic acid content may be a useful adjunct to cytologic examination (143).
Gadolinium-enhanced MRI of the neuroaxis can assist in identifying leptomeningeal metastases. When headache is the presenting feature, gadolinium-enhanced MRI examination of the brain is the initial imaging investigation, especially if signs of cranial nerve involvement are present (144, 145). If this is nondiagnostic and if the pain distribution indicates spinal involvement, the sensitivity is enhanced by performing an examination of the whole spine. There is evidence that gadolinium-enhanced spinal MRI may be positive in almost 50% of patients without clinical findings related to the spinal region and in 60% of patients with negative CSF cytology (146). Additionally, findings of contrast enhancement of the basilar cisterns, parenchymal metastases, hydrocephalus without a mass lesion, or spinal subarachnoid masses or enhancement may all have therapeutic implications (129).
Untreated leptomeningeal metastases cause progressive neurologic dysfunction at multiple sites, followed by death in 4–6 weeks. Current treatment strategies, which include radiation therapy to the area of symptomatic involvement, corticosteroids, and intraventricular or intrathecal or systemic chemotherapy, are of limited efficacy, and in general, patient outlook remains poor (133, 147, 148).
Base of Skull Metastases
Base of skull metastases are associated with well-described clinical syndromes (149), which are named according to the site of metastatic involvement: orbital, parasellar, middle fossa, jugular foramen, occipital condyle, clivus, and sphenoid sinus. Cancers of the breast, lung, and prostate are most commonly associated with this complication (149, 150), but any tumor type that metastasizes to bone may be responsible. When base of skull metastases are suspected, axial imaging with CT (including bone window settings) is the usual initial procedure (149). MRI is more sensitive for assessing soft tissue extension, and CSF analysis may be needed to exclude leptomeningeal metastases.
Orbital syndrome
Orbital metastases usually present with progressive pain in the retroorbital and supraorbital area of the affected eye. Blurred vision and diplopia may be associated complaints. Signs may include proptosis, chemosis of the involved eye, external ophthalmoparesis, ipsilateral papilledema, and decreased sensation in the ophthalmic division of the trigeminal nerve. Imaging with MRI or CT can delineate the extent of bony damage and orbital infiltration.
Parasellar syndrome
The parasellar syndrome typically presents as unilateral, supraorbital, and frontal headache, which may be associated with diplopia (151). There may be ophthalmoparesis or papilledema, and formal visual field testing may demonstrate hemianopsia or quadrantanopsia.
Middle cranial fossa syndrome
The middle cranial fossa syndrome presents with facial numbness, paresthesias, or pain, which is usually referred to the cheek or jaw (in the distribution of second or third divisions of the trigeminal nerve) (152). The pain is typically described as a dull continual ache but may also be paroxysmal or lancinating. On examination, patients may have hypsthesia in the trigeminal nerve distribution and signs of weakness in the ipsilateral muscles of mastication. Occasionally, patients have other neurologic signs, such as abducens palsy (149, 153).
Jugular foramen syndrome
The jugular foramen syndrome usually presents with hoarseness or dysphagia. Pain is usually referred to the ipsilateral ear or mastoid region and may occasionally present as glossopharyngeal neuralgia, with or without syncope (149). Pain may also be referred to the ipsilateral neck or shoulder. Neurologic signs include ipsilateral Horner syndrome, and paresis of the palate, vocal cord, sternocleidomastoid, or trapezius. Ipsilateral paresis of the tongue may also occur if the tumor extends to the region of the hypoglossal canal.
Occipital condyle syndrome
The occipital condyle syndrome presents with unilateral occipital pain that is worsened with neck flexion (154, 155). The patient may complain of neck stiffness. Pain intensity is variable but can be severe. Examination may reveal a head tilt, limited movement of the neck, and tenderness to palpation over the occipitonuchal junction. Neurologic findings may include ipsilateral hypoglossal nerve paralysis and sternocleidomastoid weakness.
Clivus syndrome
The clivus syndrome is characterized by vertex headache, which is often exacerbated by neck flexion. Lower cranial nerve (VI–XII) dysfunction follows and may become bilateral (156).
Sphenoid sinus syndrome
A sphenoid sinus metastasis often presents with bifrontal and or retro-orbital pain, which may radiate to the temporal regions (157). There may be associated features of nasal congestion and diplopia. Physical examination is often unremarkable, although unilateral or bilateral sixth nerve paresis can be present.
Painful Cranial Neuralgias
As noted, specific cranial neuralgias can occur from metastases in the base of skull or leptomeninges. They are most commonly observed in patients with prostate and lung cancer (158, 159). Invasion of the soft tissues of the head or neck or involvement of sinuses can also eventuate in such lesions. Each of these syndromes has a characteristic presentation. Early diagnosis may allow effective treatment of the underlying lesion before progressive neurologic injury occurs.
Glossopharyngeal neuralgia
Glossopharyngeal neuralgia has been reported in patients with leptomeningeal metastases (135), jugular foramen syndrome (149), or head and neck malignancies (160). This syndrome presents as severe pain in the throat or neck, which may radiate to the ear or mastoid region. Pain may be induced by swallowing. In some patients, pain is associated with sudden orthostasis and syncope (160, 161).
Trigeminal neuralgia
Trigeminal pains may be continual, paroxysmal, or lancinating. Pain that mimics classical trigeminal neuralgia can be induced by tumors in the middle or posterior fossa (153, 162, 163, 164) or by leptomeningeal metastases (134). Sometimes, pain may be caused by perineural spread without evidence of a discrete mass (165). Continual pain in a trigeminal distribution may be an early sign of acoustic neuroma (166). All patients with cancer who develop trigeminal neuralgia should be evaluated for the existence of an underlying neoplasm.
Ear and Eye Pain Syndromes
Otalgia
Otalgia is the sensation of pain in the ear, whereas referred otalgia is pain felt in the ear but originating from a nonotologic source. The rich sensory innervation of the ear derives from four cranial nerves and two cervical nerves that also supply other areas in the head, neck, thorax, and abdomen. Pain referred to the ear may originate in areas far removed from the ear itself. Otalgia may be caused by acoustic neuroma (167) and metastases to the temporal bone or infratemporal fossa (168, 169). Referred otalgia is reported among patients with carcinoma of the oropharynx or hypopharynx (170).
Uncommon Causes of Headache and Facial Pain
Headache and facial pain in patients with cancer may have many other causes. Unilateral facial pain can be the initial symptom of an ipsilateral lung tumor (175). Presumably, this referred pain is mediated by vagal afferents. Facial squamous cell carcinoma of the skin may present with facial pain due to extensive perineural invasion (176). Patients with Hodgkin’s disease may have transient episodes of neurologic dysfunction that has been likened to migraine (177). In some cases this may be a reversible posterior leukoencephalopathy syndrome (RPLS), which is characterized by headache, conscious disturbance, seizure, and cortical visual loss with neuroimaging finding of edema in the posterior regions of the brain (178).
Headache may occur with cerebral infarction or hemorrhage, which may be due to nonbacterial thrombotic endocarditis or disseminated intravascular coagulation. Headache is also the usual presentation of sagittal sinus occlusion, which may be due to tumor infiltration, hypercoagulable state, or treatment with L-asparaginase (179). Headache due to pseudotumor cerebri has also been reported to be the presentation of superior vena caval obstruction in a patient with lung cancer (180). Tumors of the sinonasal tract may present with deep facial or nasal pain (181).
Neuropathic Pains Involving the Peripheral Nervous System
Neuropathic pains involving the peripheral nervous system are common. The syndromes include painful radiculopathy, plexopathy, mononeuropathy, or peripheral neuropathy.
Painful Radiculopathy
Radiculopathy or polyradiculopathy may be caused by any process that compresses, distorts, or inflames nerve roots. Painful radiculopathy is an important presentation of epidural tumor and leptomeningeal metastases (see preceding text).
Postherpetic neuralgia
Postherpetic neuralgia is solely defined by the persistence of pain in the region of a zoster infection. Although some authors use this term if pain persists even after the lesion heals, in most cases a period of weeks to months is required before this label is used; a criterion of pain persisting beyond 2 months after healing of the lesion is recommended (182). One study suggests that postherpetic neuralgia is two to three times more frequent in the cancer population than the general population (183). In patients with postherpetic neuralgia and cancer, changes in the intensity or pattern of pain, or the development of new neurologic deficits, may indicate the possibility of local neoplasm and should be investigated (184).
Cervical Plexopathy
The ventral rami of the upper four cervical spinal nerves join to form the cervical plexus between the deep anterior and lateral muscles of the neck. Cutaneous branches emerge from the posterior border of the sternocleidomastoid. In the cancer population, plexus injury is frequently due to tumor infiltration or treatment (including surgery or radiotherapy) of neoplasms in this region (185). Tumor invasion or compression of the cervical plexus can be caused by direct extension of a primary head and neck malignancy or by neoplastic (metastatic or lymphomatous) involvement of the cervical lymph nodes (185). Pain may be experienced in the preauricular (greater auricular nerve) or postauricular (lesser and greater occipital nerves) regions or the anterior neck (transverse cutaneous and supraclavicular nerves). Pain may refer to the lateral aspect of the face or head or to the ipsilateral shoulder. The overlap in the pain referral patterns from the face and neck may relate to the close anatomic relationship between the central connections of cervical afferents and the afferents carried in the cranial nerves V, VII, IX, and X in the upper cervical spinal cord. The pain may be aching, burning, or lancinating and is often exacerbated by neck movement or swallowing. Associated features can include ipsilateral Horner syndrome or hemidiaphragmatic paralysis. The diagnosis must be distinguished from EC of the cervical spinal cord and leptomeningeal metastases. MRI or CT scan of the neck and cervical spine is usually required to evaluate the etiology of the pain.
Brachial Plexopathy
The two most common causes of brachial plexopathy in patients with cancer are tumor infiltration and radiation injury. Less common causes of painful brachial plexopathy include trauma during surgery or anesthesia, radiation-induced second neoplasms, acute brachial plexus ischemia, and paraneoplastic brachial neuritis.
Malignant brachial plexopathy
Plexus infiltration by tumor is the most prevalent cause of brachial plexopathy. Malignant brachial plexopathy is most common in patients with lymphoma, lung cancer, or breast cancer. The invading tumor usually arises from adjacent axillary, cervical, and supraclavicular lymph nodes (lymphoma and breast cancer) or from the lung (superior sulcus tumors or so-called Pancoast tumors) (186, 187, 188). Pain is nearly universal, occurring in 85% of patients, and often precedes neurologic signs or symptoms by months (187). Lower plexus involvement (C7, C8, and T1 distribution) is typical and is reflected in the pain distribution, which usually involves the elbow, medial forearm, and fourth and fifth fingers. Pain may sometimes localize to the posterior arm or elbow. Severe ache is usually reported, but patients may also experience constant or lancinating dysesthesias along the ulnar aspect of the forearm or hand.
Tumor infiltration of the upper plexus (C5-6 distribution) is less common. This lesion is characterized by pain in the shoulder girdle, lateral arm, and hand. Seventy-five percent of patients presenting with upper plexopathy subsequently develop panplexopathy, and 25% of patients present with panplexopathy (186).
Cross-sectional imaging is essential in all patients with symptoms or signs compatible with plexopathy. Although comparative data on the sensitivity and specificity of MRI and CT scan in evaluating lesions of the brachial plexus is not available, MRI is widely thought to be the best choice for evaluating the anatomy and pathology of the brachial plexus (189, 190).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree