Fig. 7.1
Frequencies of the CUP syndrome in Germany (The data was made available from the Robert Koch Institute (see [3]) and adapted from [2]). (a) Absolute CUP numbers for men (blue color) and women (red color) in Germany from 2000 to 2010. Note the significant rise from 2400 and 2800 (2000) to approximately 6100 (2010). (b) Age-standardized CUP incidence rates (DMDR) for men and women in Germany from 2000 to 2010. Note the significant increase (105 and 140 %) from 5.19 and 3.70 (2000) to 10.6 and 7.9 (2010) per 100,000 inhabitants. (c) Percentage of CUP syndromes of all new malignant diagnoses in Germany (2000–2010). Note the relative stability of the respective numbers. The proportion is on average 0.2 % lower among men than among women
7.2 Definition
CUP is defined as a histologically confirmed metastatic neoplasm for which the localization of the primary cannot be identified using standardized diagnostic procedures [4]. Currently, there is no conclusive consensus on the minimum diagnostic requirements that ultimately define a CUP. For many experts, in addition to the detailed past and present medical history of the patient and physical examination, a full blood count, chemical laboratory data, and a computerized tomography (CT) of the thorax, abdomen, and pelvis are essentially required. Further diagnostic investigations (gastroscopy, colonoscopy, panendoscopy, others) may be required depending on the clinical picture. Other image-generating methods (PET-CT, others) are also being discussed as mandatory [5].
7.3 Clinical Picture and Biology
In contrast to the typical clinical presentation of a malignant tumor, CUP shows a different temporal as well as spatial behavior, which impedes detection of the primary tumor by currently available diagnostic methods [4]. Metastases occur at an early stage and/or the pattern of metastasis is unusual or non-typical. At the time of diagnosis, more than 50 % of CUP patients have metastases in multiple locations, most frequently in the liver; lungs; brain; cervical, axillary, or inguinal lymph nodes; and in the pleural and abdominal cavity.
Following these clinical observations, basically two theories on the pathogenesis of CUP have emerged [6], which partially overlap. The first theory postulates a specific and independent biology for all CUP tumors, which allows for very early and aggressive metastatic spreading, while the primary tumor is either growing slowly or not at all (dormancy) or regresses and “disappears.” In this context, it has also been postulated that a prototypical CUP tumor does not develop gradually through premalignant intermediates, but that tumor cell clones evolve very rapidly with the capability to metastasize and possibly regress locally after metastatic colonization of distant organs. This biological process leads to a clinical picture where the tumor is already and only obvious as systemic disease at the time of diagnosis. The second theory postulates organ-specific, functionally heterogeneous mechanisms for the occurrence of a CUP. According to this theory, there is no common “one size fits all” CUP biology, but several organ-specific CUP-defining molecular makeups. However, also according to this theory, entity-specific regression phenomena and the development of a very early metastatic potency appear to play a central role [7].
7.4 Autopsy Data
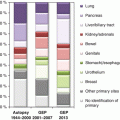
Large autopsy studies have shown that the most common primary tumors of a CUP syndrome are found in the lung and pancreas (each about 20–25 %), followed by the liver and bile ducts, colorectum, renal and adrenal glands, genitals, and stomach (each about 5–10 %) [8]. However, in these studies, a putative primary tumor could only be detected in about 60–80 % of the cases, leaving a significant percentage of CUP cases without evidence of a primary tumor even after meticulous autopsy diagnostics.
7.5 Tumor Subtypes and Prognosis
Histologically, about 90 % of the CUP cases are carcinomas; the remaining 10 % comprise mainly melanomas and sarcomas. Within the group of carcinomas, well-to-moderately differentiated adenocarcinomas (60 %) dominate over poorly differentiated adenocarcinomas (30 %). Squamous cell carcinomas and undifferentiated carcinomas are relatively rare (each about 5 %).
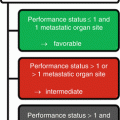
In general, median survival times of patients diagnosed with CUP are short and range between approx. 10 weeks and 17 months [1, 2, 4–9]. Only 25 % of the CUP patients survive the first year after diagnosis. The median age of patients at diagnosis is 65–70 years; CUP syndromes in children are extremely rare. From a clinical-pathological point of view, patients with CUP can be divided into a smaller subgroup with a relatively good prognosis (20 % of the patients) and in a second, larger subgroup with relatively poor prognosis [10]. The first group, which responds comparably well to chemotherapy, is quite heterogeneous and comprises patients with papillary adenocarcinoma in the peritoneal cavity, adenocarcinoma exclusively located in the axillary lymph nodes, isolated involvement of cervical or inguinal lymph nodes by squamous cell carcinoma, poorly differentiated carcinomas of the midline (midline tumor), male patients with osteoblastic metastases of adenocarcinoma and elevated PSA levels, patients with adenocarcinomas with a morphology and immunophenotype highly suggestive of colon cancer, as well as patients with a small and potentially resectable metastatic tumor.
Apart from the latter group, from a pathological point of view, the link between the tumors in this heterogeneous mixture of neoplasms is fairly obvious: although these tumors are formally and correctly classified as CUP (if a primary cannot be identified), the particular pattern of metastasis and/or tumor differentiation points toward a specific organ of origin (e.g., in the same order as above: ovarian cancer, breast cancer, squamous cell carcinoma of the head and neck, testicular germ cell tumor, prostate carcinoma, etc.). Hence, if these patients are treated according to the clinically undetectable but putative primary tumor, this group of patients will likely benefit from those chemotherapy regimens that have been established for the corresponding primaries [10].
The majority of CUP patients (80 %), however, follow an unfavorable clinical course. This applies to patients with non-intestinal-type metastases of adenocarcinomas located in the liver and other organs, multiple cerebral or multiple pulmonary/pleural metastases, evidence of non-papillary adenocarcinoma in ascites, squamous cell carcinoma in the abdomen or in the lesser pelvis, and patients with multiple osteolytic bone metastases as well as too many other very heterogeneous clinical constellations.
7.6 Aim of the Pathological Diagnosis
The primary aim of every pathological diagnosis is the most accurate and precise characterization of a tumor using histomorphology and immunohistochemical methods [10] as well as adjunct molecular approaches (see below) to guide and support optimal clinical management. In addition to a precise classification of a given tumor, the identification of clinically actionable molecular alterations by genotyping and other molecular profiling methods (e.g., transcriptomics, proteomics) will become increasingly important in the near future.
7.7 Current Principles of Conventional Pathological Diagnosis: Tissue Preparation
Whenever possible, a clinically accessible lesion of a CUP patient should be biopsied with the goal to retrieve at least a proper tissue core cylinder. Cytology specimens and cellular smears do not show the association of the tumor to its surrounding microenvironment; therefore, only cytological (cellular atypia) but not histological criteria (growth pattern, architecture) can be evaluated. The latter, however, do play a crucial role and contribute strongly to the pathological diagnosis, particularly in CUP tumors where diagnosis is challenging. Even in the tissue core biopsy setting, however, the amount of material obtained is usually small, so that on average only about 15–25 histological slides with sufficient tumor cellularity can be prepared from the standard paraffin-embedded biopsy tissue material. Since a considerable number of these tissue slides have to be used for the mandatory conventional morphological assessment and immunohistochemical staining, and subsequent slightly thicker slice preparations are necessary for morphology-controlled extraction of nucleic acids (for further molecular analysis on the mRNA, miRNA, or DNA level), the effective and efficient use of the tissue is of paramount importance. Hence, technical optimization of methodologies as well as consideration of all available and clinically relevant information (medical history of the patient, imaging, laboratorial chemical parameters) plays a crucial role in the diagnostic process. Optimized processing of tissue includes: (1) the use of immunohistochemical double stainings (see below), (2) a precisely planned initial workup procedure comprising sufficient numbers of blank slides at the first incision of the paraffin block to avoid loss of tissue by recutting, and (3) the use of well-established molecular assays for which the minimum amount of nucleic acids and the minimum tumor cell content that still yields valid data have been thoroughly determined. In addition, the use of multiplex molecular assays is clearly preferred over the use of sequential single marker molecular testing (see below).
7.8 The Diagnostic Gold Standard: Combination of Histomorphology, Immunohistochemistry, and Targeted Molecular Analysis
For the standard diagnostic workup of CUP, a gradual, synthetic diagnostic approach (Fig. 7.2) is recommended. In daily pathological routine, deviations from the gold standard approach may be necessary and meaningful in single cases. However, a stringent constructive approach must be maintained in order to get results, which are reliable, clinically useful, and fast while protecting tissue resources. An overview of the standard diagnostic steps is presented in Box 7.1.
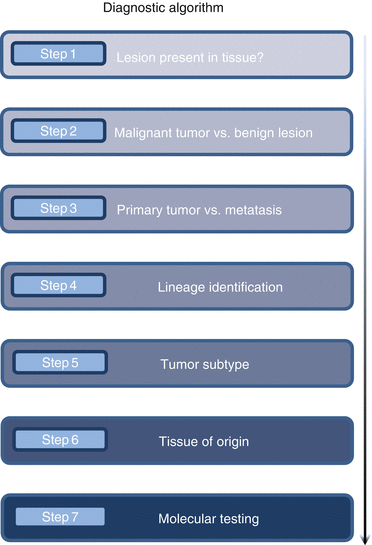

Fig. 7.2
Flowchart to visualize the initial step-by-step CUP diagnostic procedure (The figure is adapted from [2])
Box 7.1: Multistep Algorithm of Morphological CUP Diagnostics
Step 1. Histomorphological confirmation that the lesion which is clinically observed is contained in the tissue material
Step 2. Histomorphological confirmation that the lesion which is clinically observed is in fact a malignant tumor
Step 3. Differential diagnostic exclusion of a local primary tumor
Step 4. Identification of the tumor type/lineage (carcinoma, sarcoma, melanoma, lymphoma)
Step 5. Identification of the tumor subtype (e.g., adenocarcinoma, squamous cell carcinoma)
Step 6. Identification of a putative tissue of origin (organ)
Step 7. Further molecular tests for prognostication and/or prediction of therapy response
The first step of diagnosis is always based on the histomorphological evaluation of the tissue, usually stained with hematoxylin and eosin (HE) and periodic acid-Schiff (PAS). First, it is important to determine whether the clinically observed lesion is included in the biopsy. In this context, it may be necessary to level the tissue block to obtain deeper, potentially more significant tissue sections. It has been shown that in approximately 15 % of the cases, after conventional histology workup, the available tissue material is insufficient (quantitatively and/or qualitatively) for further diagnostic procedures [10]. In such cases, the clinical colleagues must be informed by the pathologist, and an additional biopsy should be obtained whenever possible. Classification of a tumor as CUP on the basis of insufficient tissue material is inadequate and may compromise the prognosis of the CUP patient.
If a lesion is identified, it has to be checked whether the lesion is a malignant tumor. This is achieved by the evaluation of conventional cytological (e.g., nuclear atypia, increased (atypical) mitoses) and histological (e.g., patterns of growth, invasion of the surrounding tissue, blood vessels, or nerves) criteria of malignancy. To determine whether the tumor is a local primary or a metastasis, histomorphology is crucial in addition to mandatory clinical information (a single lesion or multiple lesions of similar nature in the same/different location(s)). In this context, the histological findings have to be carefully integrated to obtain a precise and meaningful picture (e.g., direct association with the tissue of origin, presence of precursor lesions, spatial relationship to the organ system). For example, an adenocarcinoma in the muscular wall of the colon with direct contact to the surface epithelium and dysplastic adenoma at the borders of the tumor is almost certainly a primary, while an adenocarcinoma in deep wall layers of the colon without contact to the mucosa and without accompanying precursor lesion may be rather a metastasis. The initial meticulous morphological evaluation of the tissue is absolutely indispensable and is the basis for all types of additional typing approaches, either by immunohistochemical or molecular means.
If morphology is not definite, immunohistochemistry (IHC) is used to determine the major lineage/origin of the tumor (epithelial = carcinoma, lymphoid = lymphoma, mesenchymal = sarcoma, melanocytes = melanoma) [11] (for examples see: Fig. 7.3, graph Fig. 7.4). The primary immunohistochemical approach for CUP diagnosis is based on the combined morphological features of the tumor. If morphology is entirely unclear, the primary IHC approach comprises: (1) pancytokeratin antibodies for the detection of epithelial differentiation and (2) antibodies for detecting of melanocytic and (3) lymphoid cells. The differential diagnosis of a mesenchymal neoplasm is primarily based on the negativity for epithelial, melanocytic, and lymphoid markers, respectively, and simultaneous detection of (4) expression of the “first-line marker” vimentin. The latter, however, should be interpreted with caution as vimentin is frequently expressed in a variety of tumors including poorly differentiated carcinomas undergoing EMT [12]. Hence, integrated evaluation of the other IHC staining results is mandatory. The final diagnosis of a carcinoma, sarcoma, melanoma, or leukemia/lymphoma requires the appropriate morphology of the tumor as well as appropriate immunohistochemical results and additional genetic investigations where necessary.
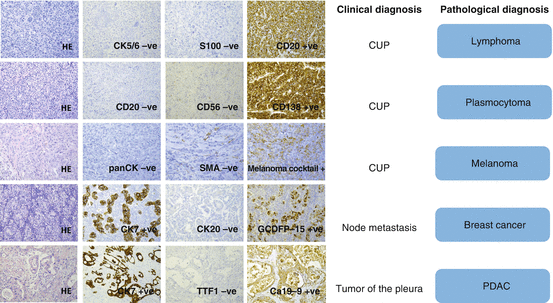
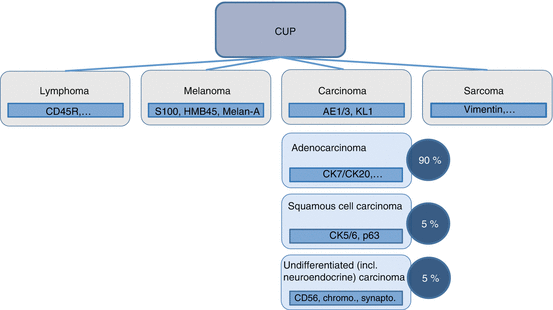

Fig. 7.3
Prototypical diagnostic examples. In these cases of essentially undifferentiated tumor, further subtyping is achieved by immunohistochemistry. CD cluster of differentiation, CK cytokeratin, GCDFP gross cystic disease fluid protein, HE hematoxylin and eosin, SMA smooth muscle actin, TTF thyroidal transcription factor, PDAC pancreatic ductal adenocarcinoma (The figure is adapted from [2].)

Fig. 7.4
Percentage of different histologies in CUP syndrome and first steps for class typing in cases with noncharacteristic morphology (The figure is adapted from [2])
In this context, some general principles apply for the definition of the tumor lineage.
In addition to conventional morphology, immunohistochemically detectable expression of keratins and organ-specific marker profiles is indicative of carcinomas.
Besides morphological criteria, subtyping of sarcomas can be achieved by a variety of markers (e.g., vimentin, actin, desmin, MyoD1, S100, CD34, CD99, c-KIT, many others) as well as genetic approaches (particularly translocation analysis; see also below) [12].
For the diagnosis of malignant melanoma, immunohistochemical detection of S100 and melan-A as well as the use of the antibody clone HMB45, which recognizes the cytoplasmic glycoprotein gp100, may be helpful. Mutation analyses (see below) may be informative [13].
For differential diagnosis of lymphatic or hematopoietic neoplasms, numerous immunohistochemically detectable cluster of differentiation (CD) antigens and other marker proteins can be used. In addition, molecular analysis (particularly cytogenetics) may help to establish the correct diagnosis [14].
7.9 Algorithms for Carcinoma Subtyping
For the differential diagnosis and subtyping of carcinomas, which comprise the largest group of CUP, morphology in conjunction with immunohistochemical marker panels plays an important role (step 5, Box 7.1). In an ideal setting, these markers are used in diagnostically meaningful double staining combinations to spare tissue slides (Fig. 7.5). In some cases, this method allows to determine the tissue of origin (step 6, Box 7.1). To identify squamoid neoplasms, particularly squamous differentiation markers such as high molecular weight cytokeratins (e.g., CK5/6) are used. While the expression of cytokeratins 5 and 6 is very sensitive, but not specific for squamous cell carcinoma, additional detection of nuclear p40 and/or p63 staining yields a specificity of about 90 % and sensitivity of about 70 % [16]. Squamous cell carcinomas are usually negative for cytokeratins 7 and 20 – two cytokeratins that are often expressed by adenocarcinomas. Among others, determination of neuroendocrine lineage is achieved by the detection of the very specific antigens chromogranin A and synaptophysin as well as CD56 (neural cell adhesion molecule, N-CAM), which is very sensitive but less specific than the two aforementioned markers [17]. A fourth antigen indicating neuroendocrine differentiation, NSE (neuron-specific enolase), is a very sensitive but even less specific marker, so that its diagnostic utility and significance are rather limited. Adenocarcinomas (and some other epithelial neoplasms), which form the largest group of CUPs, can initially be classified into four broad diagnostic categories on the basis of the expression of the two cytokeratins CK7 and CK20 (Fig. 7.6) [1, 2, 4–14, 16–18].
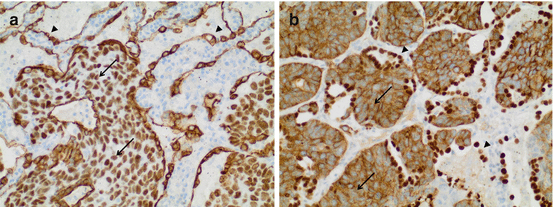
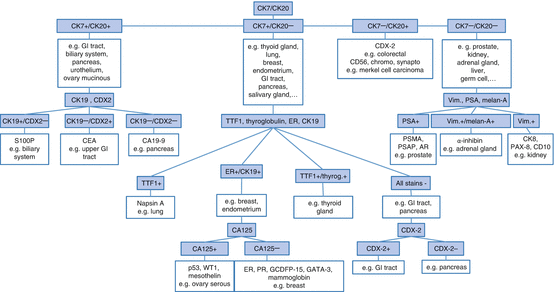

Fig. 7.5
Tissue-sparing combinations of antibodies used in simultaneous double staining procedures. (a) Squamous cell carcinoma of the lung. Staining for p63 (nuclear) is combined with staining for CK7 (cytoplasmic). Note p63 positivity and CK7 negativity within the tumor (arrows), while the epithelial lining of alveolar walls shows CK7 positivity (arrowheads). (b) The same squamous cell carcinoma of the lung stained with a different antibody cocktail comprising CK5/6 (cytoplasmic) as well as TTF1 (nuclear) antibodies. Note that the tumor stains for CK 5/6 (arrows) but not TTF1, while the alveolar lining is positive for TTF1 (arrowheads). In both cases, an antibody against a cytoplasmic protein is combined with an antibody directed against a nuclear protein. In most cases, only one of the two proteins is expressed due to intelligent combination of antibodies (The figure is adapted from [15])

Fig. 7.6
Decision tree for immunohistochemical subtyping of adenocarcinomas (Adapted from [2, 4–7]). The presented entity and marker combinations are only examples and not exhaustive; the marker combinations are also not completely specific for these tumor entities; for further details, please refer to the main text
1.
CK20+/CK7+: e.g., gastrointestinal adenocarcinoma, transitional cell carcinoma andmucinous ovarian tumors
2.
CK20+/CK7−: e.g., colorectal adenocarcinoma and neuroendocrine tumors of Merkel cell type
3.
CK20−/CK7−: e.g., prostate carcinoma, hepatocellular carcinoma (HCC), clear cell renal cell carcinomas (RCC), small cell carcinoma
4.
CK20−/CK7+: e.g., pancreatic cancer, bile duct carcinoma, breast carcinoma, pulmonary adenocarcinoma, salivary gland carcinoma, and endometrial carcinoma
It should be noted, however, that this list is by no means exhaustive and that aberrant immunophenotypes that significantly hamper diagnostic accuracy are not rare in CUP disease.
After initial classification, markers which facilitate determination of the organ of origin are used for further classification of adenocarcinomas. For prostate carcinomas, this includes immunohistochemical detection of the androgen receptor, PSMA and PSA [19], for pulmonary adenocarcinoma the markers napsin A and TTF1 [20], and for colorectal/gastrointestinal adenocarcinomas CDX2 (caudal-type homeobox transcription factor-2) [19]. The typing of breast cancer can be achieved through detection of the antigens GCDFP-15, mammaglobin, and GATA3 as well as through the detection of estrogen receptor (ER) and progesterone receptor (PR) expression. Many adenocarcinomas of the ovary are positive for CA-125, mesothelin, PAX8, and WT1 (nuclear) as well as ER [21]. For diagnosis of renal cell carcinoma, the coexpression of vimentin, CD10, PAX8, and cytokeratins is corroborative. In addition, in some cases, the expression of the brush-border proteins of the proximal renal tubule can be demonstrated by the RCC antibody [22]. Thyroid carcinomas show a positive immune reaction for TTF1 and thyroglobulin [22], while alpha-inhibin and melan-A are considered as established markers for adrenal carcinoma [23]. Hepatocellular carcinomas are identified by the presence of OCH1E5 as well as arginase-1 expression [24, 25]. However, although many efforts have been made to identify organ-specific markers (i.e., proteins/antigens), it is important to note that for a variety of common neoplasms, specific markers are still missing (e.g., cholangiocarcinoma, pancreatic ductal carcinoma, gastric carcinoma), and aberrant expression patterns may occur.
For squamous cell carcinoma, site-specific markers are entirely lacking. Metastatic squamous cell carcinoma of the head and neck region cannot be distinguished from squamous tumors originating from the esophagus, urinary system, uterine cervix, anus, skin, and other rare primary locations by conventional histology and immunohistochemistry. Currently, the only exception is the detection of HPV genome, which strongly points toward a primary from either the oropharyngeal region or the uterine cervix. For example, HPV analysis has been successfully used to delineate primary pulmonary squamous cell carcinomas from lung metastasis of squamous cell carcinomas of the head and neck region [26]. This is a prototypical example where a single molecular analysis may contribute valuable information to CUP diagnostics. HPV detection in this context can either be done by in situ as well as PCR-based methods (Fig. 7.7).
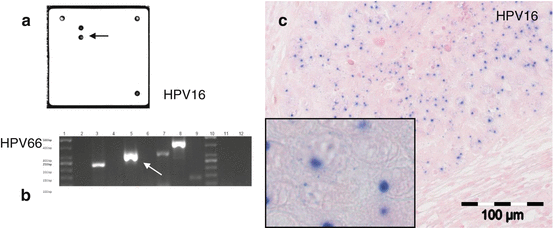

Fig. 7.7
Different methods of detecting HPV in tumor tissue (in this case in an oropharyngeal squamous cell carcinoma). (a) Detection of HPV and further subtyping with specific hybridization probes in a small-scale array format. This diagnostic procedure is performed with nucleic acid extracts from tumor tissue. (b) HPV detection and subtyping by multiplex PCR and gel electrophoresis from nucleic acid extracts. (c) In situ detection of HPV genomic material by chromogene in situ hybridization (CISH) (The figure is adapted from [26])
Common immunohistochemical markers for organ typing are exemplarily shown in Table 7.1. However, the authors would like to emphasize that these markers achieve diagnostic significance exclusively and only in conjunction with the histomorphological findings. Moreover, the immunohistochemical characterization of a tumor reaches sufficient specificity only by the interpretation of positive and negative staining results of the entire antibody panel (i.e., the entire staining pattern), not by isolated consideration of single proteins.
Table 7.1
Markers for immunohistochemical typing of CUP
Identification of the tumor lineage | |
Carcinoma
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |