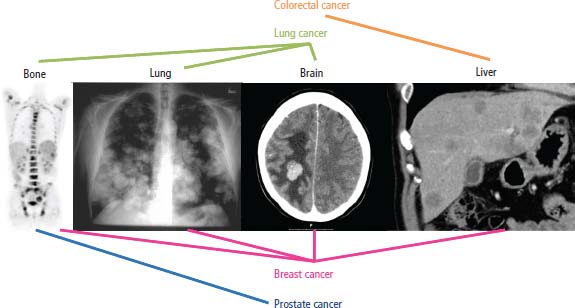
37 For most patients who present with metastatic disease, routine examination and investigation will quickly disclose the underlying primary tumour. Occasionally, the primary tumour may be more elusive, and a number of clinical, histopathological and serological clues may help to establish its site. For 3–5% of patients, however, the primary site remains undisclosed because it is too small to be detected or has regressed. The usual histological diagnosis in these patients with a cancer of unknown primary (CUP) site is adenocarcinoma or poorly differentiated carcinoma. The benefits of establishing the primary site include: diagnosing treatable disease (Table 37.1) In 2010, a total of 9762 people were diagnosed with CUP and 10,812 people died of CUP. That more people died of CUP than were diagnosed with it is chiefly a consequence of the vagaries of data recording. Although CUP accounts for 3% of cancers diagnosed in the United Kingdom, it causes nearly 7% of cancer deaths. Over the last decade, the incidence of CUP has declined markedly – by 34% in women and 39% in men. This probably, in part, reflects improvements in imaging and immunohistochemical staining to establish a primary tumour site. The incidence of CUP rises with age and whilst it accounts for 3% of cancers overall, 7% of cancers in those over 85 years old are CUP. Different tumours follow different patterns of metastatic spread. This may be related to chemokine and chemokine receptor expression by tumours and stromal cells (see Box 1.4 and Box 2.2). The most common sites of metastases are shown in Table 37.2 and Figure 37.1. Table 37.1 Treatable unknown primary diagnoses Up to a quarter of solid tumours develop parenchymal brain metastases. Parenchymal brain secondaries that may occur with any solid tumour are usually treated with whole-brain radiotherapy, although surgery may be considered for patients with solitary brain metastases and limited systemic disease (Figures 13.8, 33.8 and 37.1). Carcinomatous meningitis is less common and presents with multiple, anatomically distant, cranial and spinal root neuropathies. The diagnosis may be confirmed by finding malignant cells in the cerebrospinal fluid. Treatment usually involves a combination of intrathecal chemotherapy and craniospinal radiotherapy. Carcinomatous meningitis most frequently occurs with leukaemias and lymphomas and occasionally with breast cancer. Table 37.2 Table of most common sites of cancer metastases Bone metastases are a major source of morbidity in patients with cancer and often have a prolonged course. Bone metastases cause pain, reduced mobility, pathological fractures, hypercalcaemia, myelosuppression and nerve compression syndromes. The tumours that commonly metastasize to bone are lung, breast, prostate, renal and thyroid tumours and sarcomas. Metastases usually occur in the axial skeleton, femur or humerus. If they are found elsewhere, then renal cancer and melanoma should be considered as possible primary tumour sites. Most bone metastases are lucent, lytic lesions; occasionally dense, sclerotic deposits are seen in prostate, breast, carcinoid tumours and Hodgkin’s disease. The diagnosis of bone metastases is rarely complicated. The differential diagnosis is outlined in Table 37.3 (Figures 3.4, 3.6, 5.6, 7.3, 12.1, 14.3, 14.4, 14.5, 26.4, 31.2, 31.5, 37.1, 40.6d, 40.7, 40.8 and 40.9). Figure 37.1 Common patterns of metastatic spread for the four most frequent cancers. Table 37.3 Differential diagnosis of bone metastases The lungs are the second most common site for metastases via haematogenous spread. Lung, breast, renal, thyroid, sarcoma and germ cell tumours commonly metastasize to the lung. Surgical resection of pulmonary metastases is occasionally undertaken where the primary site is controlled and the lungs are the sole site of metastasis (see Figures 15.1 and 37.1). Of all patients with liver metastases Hepatic resection for patients with up to three metastases from colorectal cancer results in 5-year survivals of 30% and is the best treatment available for selected patients (see Figures 9.1, 23.2 and 37.1). Eighty per cent of malignant pleural effusions are due to lung and breast cancers, lymphoma and leukaemia. Malignant pericardial effusion is rarer than pleural effusions; breast and lung cancers account for 75%. Metastases to the heart and pericardium are 40 times more common than primary tumours at these sites, but only 15% will develop tamponade. Malignant ascites is a common complication of ovarian, pancreatic, colorectal and gastric cancers and lymphoma. Measures for long-term control of malignant effusions include sclerosis with talc, bleomycin or tetracycline for pleural effusions, drainage by pericardial window for pericardial effusions and peritoneovenous shunts for malignant ascites (see Figure 40.20 and 40.21). Five highly treatable subsets of unknown primary sites have been identified, which have more favourable outcomes and require distinct management: Unfortunately, the majority of unknown primary tumours do not fit into any of these subsets and the response rates to chemotherapy are below 20%. These responses are usually of brief duration, with limited impact on overall survival. The median survival is under 12 months. The exception to this rule is in the group of patients who are under 45 years old. In this group, treatment with BEP (bleomycin, etoposide and cisplatin) or a taxane combination is worthwhile. For this group of patients, 50% survive in excess of 2 years despite the fact that the tumours do not have the characteristics of germ cell cancers and do not stain positively for HCG or AFP. Table 37.4 Immunohistochemical staining profiles in CUP that suggest a primary tumour site Table 37.5 The most common serum tumour markers and their uses HCC, hepatocellular carcinoma; HLA, human leucocyte antigen; TB, tuberculosis; UC, ulcerative colitis; MEN, multiple endocrine neoplasia. The histopathological characterization of unknown primaries to establish their origin includes a number of techniques: light microscopy, immunocytochemical staining (Table 37.4), immunophenotyping, electron microscopy, cytogenetics and molecular analysis. These are described in detail in Chapter 3. There are numerous studies investigating the role of molecular diagnostics, including microarray-based gene expression profiling of CUP tumours. Tumour markers are proteins produced by cancers that are detectable in the blood of patients. Ideally, serum tumour markers should be quick and cheap to measure, have high sensitivity (of more than 50%) and specificity (over 95%) and yield a high predictive value of positive (PPV) and negative (NPV) results. Under these circumstances, tumour markers may be used for population screening, diagnosis, as prognostic factors, for monitoring treatment, diagnosing remission and detecting relapse and for imaging metastases. A large number of serum tumour markers are available, and each may be valuable for any of screening, diagnosis, prognostication and monitoring treatment (Table 37.5). There is a worrying tendency to over-investigate patients with unknown primary cancer while at the same time ignoring their palliative care needs. So often the greater the eminence and number of consultants whose advice is sought, the larger the number of esoteric investigations ordered, and the less well the patient and their family are informed. Investigations should be restricted to those that will alter clinical management. It is estimated that in the absence of a localizing symptom, extensive radiological investigation leads to the identification of a primary site in less than 5% of all patients. The prognosis is generally poor, with a median survival of 3–4 months. Less than 25% of patients survive to 1 year and less than 10% are alive after 5 years. The site of the primary is usually on the same side of the diaphragm as the metastases, and 75% of tumours are infradiaphragmatic; of the 25% that arise above the diaphragm, nearly all arise from the lung. Where identified, the most common primary sites, in order of frequency, are lung, pancreas, liver, colorectal, stomach, kidney, prostate, ovary, breast, lymphoid and testis. A good performance status is the most important predictor of survival, while extensive weight loss and older age are adverse prognostic factors. With the exception of the five clinical syndromes listed above, treatment other than symptom palliation is rarely appropriate. Case Study: A tired retired engineer.
Cancer of unknown primary
Epidemiology and pathogenesis
Clinical sites of metastatic spread
Chemosensitive
Hormone-sensitive
tumours
tumours
Non-Hodgkin’s lymphoma
Breast cancer
Germ cell tumours
Prostate cancer
Neuroendocrine tumours (including small cell lung cancer)
Endometrial cancer
Ovarian cancer
Thyroid cancer
Brain and meningeal metastases
Site
First metastatic location incidence
Incidence of involvement at presentation
Lymph node
26%
41%
Lung
17%
27%
Bone
15%
29%
Liver
11%
34%
Brain
8%
6%
Pleura
7%
11%
Skin
5%
4%
Peritoneum
4%
9%
Adrenal gland
–
6%
Bone marrow
–
3%
Bone metastases
Diagnosis
Pain
Site
Age
X-ray
Bone scan, CT/MRI
Biochemistry
Metastases
Common
Axial skeleton
Any
Discrete lesions, pathological fracture, loss of vertebral pedicles
Soft tissue extension on MRI/CT
Raised ALP and Ca
Degenerative disease
Common
Limbs
Old
Symmetrical
Symmetrical uptake on bone scan
Normal
Osteoporosis
Painless (unless pathological fracture)
Vertebrae
Old (female)
Osteopenia
Normal bone scan/MRI
Normal
Paget’s disease
Painless
Skull (often)
Old
Expanded sclerotic bones
Diffusely hot bone scan
Raised ALP and urinary hydroxyproline
Traumatic fracture
Always
Ribs
Any
Fracture
Intense linear uptake on bone scan
Normal
ALP, alkaline phosphatase; Ca, calcium; CT, computed tomography; MRI, magnetic resonance imaging.
Lung metastases
Liver metastases
Malignant effusions
Clinical CUP syndromes
Primary tumour site
Immunohistochemical profile
Lung (adenocarcinoma, large cell)
CK7+, CK20−, TTF-1+
Lung neuroendocrine (small cell)
Chromogranin+, synaptophysin+, TTF-1+
Colorectal
CK7−, CK20+, CDX-2+
Breast
CK7+, ER+, GCDFP-15+, mammoglobulin+
Prostate
CK7−, CK20−, PSA+
Ovary
CK7+, ER+, WT-1+
Melanoma
S100+. melan-A+, HMB45+
Renal
RCC+, vimentin+, CD10+, PAX-8+
Liver
Hepar+, CD10+, CD13+
Germ cell
PLAP+, OCT-4+
Adrenal
Alpha-inhibin+, melan-A+
Thyroid (follicular, papillary)
TTF-1+, thyroglobuin+
CK, cytokeratin; TTF, thyroid transcription factor; CDX, caudal type homeobox; ER, estrogen receptor; GCDFP, gross cystic disease fluid protein; PSA, prostate-specific antigen; WT, Wilms’ tumour; HMB, human melanoma black; RCC, renal cell carcinoma; CD, cluster differentiation; PAX, paired box; PLAP, placental alkaline phosphatase; OCT, octamer binding transcription factor.
Name
Natural occurrence
Tumour
Comments
Screening
Diagnosis
Prognosis
Follow-up
Carcino embryonic antigen (CEA)
Glycoprotein found in intestinal mucosa during embryonic and foetal life
Colorectal cancer (especially liver metastases), gastric, breast and lung cancers
Elevated in smokers’ cirrhosis, chronic hepatitis, UC, Crohn’s, pneumonia and TB (usually <10 ng/mL)
No
Yes
Yes
Yes
Alpha-foetoprotein (AFP)
Glycoprotein found in yolk sac and foetal liver
Germ cell tumours (GCTs) (80% non-seminomatous GCTs), hepatocellular cancer (50%), neural tube defects, Down’s pregnancies
Role in screening in pregnancy not cancer Only prognostic for GCT not HCC Transient increase in liver diseases
No
Yes
Yes
Yes
Prostate-specific antigen (PSA)
Glycoprotein member of human kallikrein gene family; PSA is a serine protease that liquefies semen in excretory ducts of prostate
Prostate cancer (95%), also benign prostatic hypertrophy and prostatitis (usually <10 ng/mL)
Tissue specific but not tumour specific, although a level of >10 ng/mL is 90% specific for cancer
*
Yes
No
Yes
Cancer antigen 125 (CA-125)
Differentiation antigen of coelomic epithelium (Muller’s duct)
Ovarian epithelial cancer (75%), also gastrointestinal, lung and breast cancers
Raised in cirrhosis, chronic pancreatitis, autoimmune diseases and any cause of ascites
*
Yes
No
Yes
Human chorionic gondadotrophin (HCG)
Glycoprotein hormone, 14 kD α subunit and 24 kD β subunit from placental syncytiotrophoblasts
Choriocarcinoma (100%), hydatidiform moles (97%), non-seminomatous GCT (50–80%), seminoma (15%)
Screening post-hydatidiform mole for trophoblastic tumours, also used to follow pregnancies and diagnose ectopic pregnancies
Yes
Yes
Yes
Yes
Calcitonin
32 amino acid peptide from C cells of thyroid
Medullary cell carcinoma of thyroid
Screening test in MEN 2
Yes
Yes
Yes
Yes
Beta-2-microglobulin
Part of HLA common fragment present on surface of lymphocytes, macrophages and some epithelial cells
Non-Hodgkin’s lymphoma, myeloma
Elevated in autoimmune disease, renal glomerular disease
No
No
Yes
Yes
Thyroglobulin
Matrix protein for thyroid hormone synthesis in normal thyroid follicles
Papillary and follicular thyroid cancer
No
Yes
No
Yes
Placental alkaline phosphatase (PLAP)
Isoenzyme of alkaline phosphatase
Seminoma and ovarian dysgerminoma (50%)
No
Yes
No
Yes
*See Part 3.
Histopathological characterization
Use of tumour markers
Approach to investigation of metastatic disease to establish primary site
 ONLINE RESOURCE
ONLINE RESOURCE
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


