52 Cancer of the Vulva
The middle third of the 20th century witnessed remarkable progress in the surgical treatment of vulvar cancer. During the 1940s and 1950s, radical vulvectomy, in continuity with bilateral inguinal, femoral, and selective pelvic lymphadenectomy, emerged as the standard for operative therapy and succeeded in more than doubling the cure rates previously obtained by less-comprehensive surgical strategies.1–4 Initially, this operation was accompanied by substantial risk of perioperative mortality as well as long, difficult periods of recuperation. Refinements in surgical technique, including primary closure of the perineal wound, shortened the duration of hospitalization and recovery. Reported at rates of 6% to 19% by the pioneers of radical vulvar surgery,2,5 total operative and perioperative deaths had declined to a rate less than 4% by the 1970s,6,7 and select patients with locally extensive disease were being treated by anterior, posterior, or total pelvic exenteration, with salvage rates exceeding 50% when regional nodes were found to be free of metastatic involvement.8 When inguino-femoral nodes were found to contain metastatic deposits, node dissection was often extended cephalad above the inguinal ligaments to encompass ipsilateral or bilateral pelvic lymphadenectomy. Radiation was infrequently used and largely restricted to palliative treatment. In retrospect, radiotherapy was administered using naive dose and fractionation schedules as well as techniques of limited sophistication.9–11 The late sequelae associated with excess dose to the vulva are shown in Figs. 52-1 and 52-2.
The past 35 years have witnessed a series of remarkable transformations in the treatment of this disease. Because of the success of radical vulvar surgery, substantial numbers of patients operated on and followed up on in large referral centers became available for the assessment of treatment outcomes, including chronic morbidities of surgical therapy. Leg lymphedema following radical groin and pelvic node dissection may be temporary, lasting a year or longer, or may be permanent, massive, and disabling.6,12 Pelvic relaxation, organ prolapse, and urinary incontinence can develop in some patients when removal of >1 cm of the distal urethra or a portion of the lower vagina is required. Significant vaginal introital stenosis can occur. Absence of the vulva and venereal fat can have the functional consequence of shortening the effective vaginal depth and removing a protective cushion from the pubic arch, contributing to dyspareunia in many patients. Clitorectomy will profoundly diminish capacity for arousal and climax. The psychosexual consequences of vulvectomy are frequently devastating.13–15
The clinical extent of vulvar cancer at the time of diagnosis may be decreasing, possibly because of enlightened attitudes toward the pathogenesis of the disease, diminished social stigma associated with the diagnosis, and improved access to health care resources. Among operable patients undergoing radical groin dissection, the frequency of lymph node metastasis fell from 50% to 60% at mid-century3,4 to 30% in surgical literature from the 1980s.16–20 Younger, sexually active patients are being diagnosed with preinvasive disease or unifocal disease of minimal volume and depth of invasion, often with regional nodes free of metastatic contamination.21 Modifications in the surgical approach to vulvar cancer have been provoked by concerns about the chronic morbidities of ablative surgery in patients with disease of limited extent. In select individuals, limited vulvar and groin surgery can accomplish equivalent cancer control as more extensive surgery, with less functional loss and mutilation.22–24 Judicious use of adjuvant postoperative radiation has reduced regional relapse, improved survival, and decreased the indications for pelvic lymphadenectomy.25
Planned in coordination with surgery of reduced extent, preoperative or postoperative radiation (often done with concurrent chemotherapy) has permitted salvage in patients who would otherwise have required exenterative surgery for cure, thus, preserving sexual, urinary, and anorectal function historically often sacrificed in the era of radical surgical monotherapy.26–39 Extrapolating from the success achieved in fashioning effective conservative therapy for patients with carcinoma of the anal canal, synchronous chemotherapy and radiation in reduced dose have accomplished curative nonsurgical management of some patients with massive, unresectable vulvar disease and of other patients judged too frail for extensive surgical intervention.40–43 A proliferation of management options now permits treatment responsibly tailored to the initial volume and extent of disease, the presence or absence of defined clinical and histopathologic prognostic factors, and the age, anatomy, personality, and priorities of the individual patient. This chapter reviews essential elements of these contemporary therapeutic strategies.
Epidemiology
Vulvar malignancies constitute less than 5% of female genital tract cancer, and only 1% to 2% of cancer in women. Historically, cancer of the vulva was most commonly observed in the seventh and eighth decades of life, with age-specific incidence rates in the United States rising steeply at age 70 and continuing to rise beyond age 80.44 Recent trends suggest that the average age of patients with vulvar cancer may be decreasing consequent to more common exposure to oncogenic serotypes of the human papilloma virus (HPV).45 The coincidence of vulvar intraepithelial neoplasia (VIN) or invasive squamous carcinoma of the vulva with in situ or invasive epidermoid carcinoma of the cervix has long been noted, and synchronous or sequential (usually antecedent) cervical lesions may be present in as many as 20% of women with primary vulvar lesions, suggesting a common etiology in at least some patients.46–52 Following conservative local excision, local-regional cancer regrowth, sometimes at anatomically distinct structures within conserved vulvar tissues and therefore plausibly representing metachronous appearance of independent primaries, lends credence to the so-called “field change” model of oncogenesis. Additional risk factors for the development of invasive vulvar cancer include a history of condyloma acuminatum, VIN, smoking, alcohol consumption, and chronic vulvar dystrophies, particularly lichen sclerosis.53–58
A rising incidence of VIN and reports of invasive vulvar cancer in young patients may reflect changing sexual mores and transmission of oncogenic serotypes of HPV. Detection of HPV in VIN or invasive disease is most common in young patients and is less frequent as a function of advancing age.58,59 The natural history of VIN is not well understood. Less than 10% of untreated VIN will progress to cancer with an average time to progression of 55 months.60 In general, VIN is treated with local excision. In contrast to surgical excision for invasive cancer, excision for VIN needs to be superficial only, to a depth of 1 mm in non–hair-bearing skin and 2 mm in hair-bearing skin (to include hair follicles which may be involved with VIN). Because VIN-3 may be associated with a clinically occult invasive component in up to 20% of cases,61 care must be exercised when employing treatment techniques, such as laser vaporization, which do not include full-thickness excision of the lesion. Recurrence rates after local excision depend on achieving negative surgical margins, as well as risk factors such as tobacco smoking and immunosuppression. Patients with multifocal recurrence or multiple metachronous recurrences may be treated with topical 5-fluorouracil (5-FU) cream or carbon dioxide (CO2) laser ablation rather than repeat local excisions. Patients infected with HIV are at particular risk for recurrent disease and may be treated with weekly prophylactic topical 5-FU cream.
Invasive vulvar cancer has been reported in young patients with both naturally occurring and iatrogenic immune compromise.62–66 Patients infected with HIV are more likely to have HPV infection than are HIV-uninfected women and may be more vulnerable to development of VIN than are HIV-negative controls. VIN may be more difficult to clear in HIV-infected women and may progress to invasive disease with greater rapidity.67,68 Recurrence rates for both VIN and invasive disease are higher in HIV-infected women.69
Vulvar carcinoma frequently coexists with vulvar dystrophy, particularly in older women.70,71 However, it is unknown whether lesions such as lichen sclerosis and typical or atypical hyperplastic dystrophy are true precursor lesions. Study of grossly normal genital skin and vulvar lichen sclerosis has revealed significantly higher degrees of single base instability in lichen sclerosis which may predispose to malignant transformation in some patients.72 Cancers in these older patients are less commonly associated with HPV infection, leading to the hypothesis that patients with vulvar cancer may be segregated into two broad groups: an older population with cancer arising in association with vulvar dystrophy and less likely to be associated with HPV, and a younger population with HPV-associated tumors frequently adjacent to areas of VIN.59,73
Anatomy
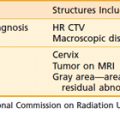
The vulva is composed of the mons veneris; labia majora; labia minora; clitoris, and vulvar vestibule, which harbors the urethral meatus and is bounded by the vaginal introitus (Fig. 52-3). The labia, limited laterally by the labiocrural folds, are the female analogue of the male scrotum and are pigmented and covered by hair lateral to the crests of the labia majora. Medially, the skin of the labia majora is smooth and hairless, with underlying sebaceous glands. Between the labia majora lie the hairless labia minora, whose medial skin also contains multiple sebaceous glands. Coursing posteromedially, the labia minora taper and join in a fold called the posterior fourchette. Anteriorly, the labia minora taper, then split to envelop the clitoris, fusing in front of the clitoris to form the prepuce (clitoral hood), and behind the clitoris to form the frenulum. The clitoris, an erectile organ analogous to the penis in the male, is composed of the glans and a body consisting of two corpora cavernosa that diverge into the two crura which lie attached to the undersurface of the pubic rami. The paired, mucus-secreting Bartholin glands lie deep to the posterior labia majora and drain by a simple duct lined by cuboidal and transitional epithelium to the vulvar vestibule between the hymenal membrane and the medial borders of the labia minora. Cancers arising in the Bartholin complex are conventionally categorized and treated as vulvar cancers.
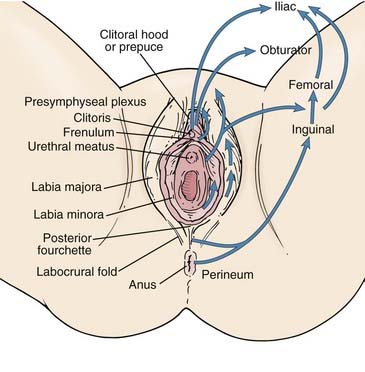
FIGURE 52-3 • Surface anatomy of the vulva and vulvar lymphatic flow.
(From Russell AH: Vulvar and vaginal carcinoma. In Gunderson LL, Tepper JE, eds: Clinical radiation oncology, ed 2, Philadelphia, 2007, Elsevier/Churchill Livingstone, p 1388.)
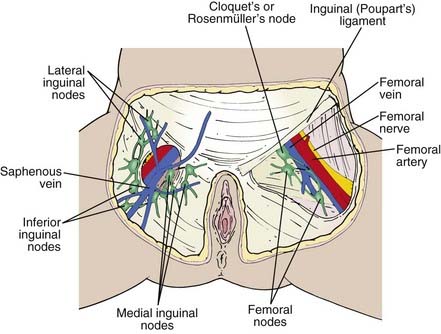
The lymphatics of the vulva are constituted by a network that covers the entire labia minora, fourchette, prepuce, and distal vagina below the hymenal membrane. These coalesce anteriorly, forming larger trunks, which run lateral to the clitoris to the mons veneris, acquiring tributaries from the lymphatics of the labia majora, which run in a parallel fashion anteriorly from the perineal body (see Fig. 52-3). The vulvar lymphatics run through the vulva and do not cross the labiocrural fold. The lymphatics of the perineum, however, course lateral to the labiocrural fold through the superficial tissues of the upper medial thigh. In the treatment of patients with advanced vulvar cancer that extends beyond the vulva to the perineal skin or anus, these more lateral channels must be addressed. Similarly, extension of an advanced vulvovaginal cancer along the vaginal canal proximal to the hymenal ring implies attention to the potential direct pelvic lymphatic flow of the middle and upper vagina. At the mons veneris, the vulvar lymphatic trunks deviate laterally to the primary regional nodes, the ipsilateral or contralateral inguinal nodes. Study of the localization of radiolabeled tracer in regional lymph nodes after focal injection of discrete sites in the vulva and on the perineum reveals that the lymphatic drainage of the perineum, clitoris, and anterior labia minora is bilateral, whereas the lymph flow from well-lateralized sites in the vulva is, predominantly, to the ipsilateral groin. From the superficial inguinal nodes, secondary lymphatic drainage is through the cribriform fascia to the deep inguinal or femoral nodes, with subsequent tertiary flow under the inguinal ligaments to the deep pelvic (external iliac and obturator) nodes (see Fig 52-3). The node of Cloquet, also called Rosenmüller’s node, is the most cephalad of the femoral nodes, often lying in the femoral canal below Poupart’s (inguinal) ligament. Metastasis to Cloquet’s node is considered a herald of pelvic node contamination and a harbinger of distant dissemination.
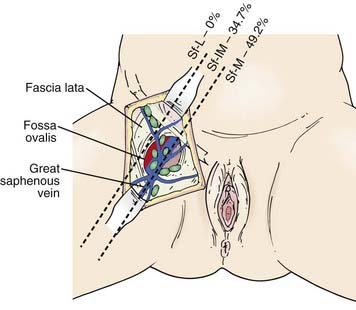
The subsurface anatomy of the groins as revealed by superficial and deep groin node dissections is depicted in Fig. 52-4. Sentinel lymph nodes have been extensively investigated in patients with vulvar cancer using blue dye technique and/or lymphoscintography. In a study of 59 patients with invasive vulvar cancers <4 cm in size who underwent anatomical localization of the sentinel nodes, 118 sentinel nodes were identified from 82 groins. Of these, 83.9% were in superficial groin nodes. Importantly, 16.1% were located in deep femoral nodes. No sentinel nodes were located in the outer third of the groin lateral to the femoral vessels (Figs. 52-5 and 52-6).74 These findings may be useful both with respect to planning the extent of groin surgery as well as the anatomic boundaries of radiation treatment volumes and prescription depth for elective groin irradiation.
FIGURE 52-5 • Location of sentinel nodes. Pf, Deep nodes—profundum.
(From Rob L, Robova H, Pluta M, et al: Further data on sentinel lymph node mapping in vulvar cancer by blue dye and radiocolloid Tc99, Int J Gynecol Oncol 17:147–153.)
Pathology
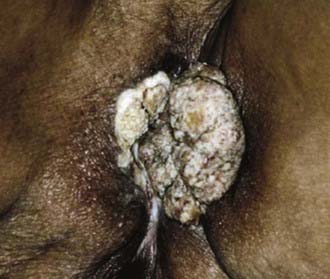
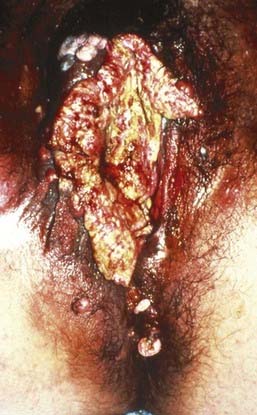
Malignant melanoma, adult and embryonal sarcomas of varied histologies, Merkel cell tumors, histiocytosis X, Kaposi’s sarcoma, hemangiopericytoma, lymphoma, eccrine carcinoma, primary breast cancers, basal cell carcinomas, adenocarcinomas, and adenoid cystic carcinomas (usually arising within the Bartholin glands), as well as other histologic tumor types have all been reported as primary malignancies arising from the vulva.75 However, more than 85% of invasive vulvar malignancies are epidermoid carcinomas76 leading to the common practice of using the terms squamous carcinoma of the vulva and vulvar carcinoma synonymously. Because of distinctive histopathology and characteristic clinical behaviors, it is important to recognize verrucous carcinoma (Fig. 52-7)77–82 and so-called “spray” pattern carcinomas (Fig. 52-8)83–85 as variants of squamous carcinoma with characteristic clinical behaviors. Verrucous cancer may resemble cauliflower in gross appearance, with a predominantly exophytic pattern of growth often accompanied by exuberant hyperkeratosis (Fig. 52-9). Histologically characterized by well-differentiated cells with a broad, pushing interface between cancer and surrounding stroma, verrucous cancer uncommonly metastasizes to regional lymph nodes, even when quite large, but often recurs locally after surgical therapy. Squamous cancers with finger-like microscopic projections, or a “spray” histologic appearance, often have a trabecular architecture of poorly differentiated cells with separate islands or discontiguous satellites of tumor cells in proximity to the infiltrative interface between the main tumor mass and surrounding stroma. These cancers may metastasize to regional nodes even when quite small and may recur at the skin margins of surgical resection, as well as relapsing distantly.
Paget’s disease of the vulva is a cutaneous change morphologically similar to Paget’s disease of the breast (nipple). Characterized histologically by large, vacuolated pathognomonic Paget’s cells with bluish cytoplasm located in the basal layer of the vulvar epithelium, Paget’s disease presents clinically as an eczematoid “weeping” lesion that is intensely pruritic. In many cases Paget’s disease may be treated with topical medications including steroids for months before a correct diagnosis confirmed by biopsy. Paget’s disease of the vulva is associated with an underlying in situ or invasive neoplasm in approximately 10% of cases. Neoplasms associated with Paget’s disease include adnexal apocrine skin carcinomas, carcinomas of Bartholin’s glands, and cancers of the urethra, bladder, vagina, cervix, endometrium, and rectum. Treatment is surgical, with cutaneous recurrences common. Negative surgical margins are difficult to obtain, because “Pagetoid” changes may extend microscopically in the epidermis, well beyond the margins of what can be appreciated on clinical examination. Negative surgical margins have only limited correlation with freedom from local relapse. Micrographic (Mohs) surgical technique has been advocated for Paget’s disease as a means to achieve negative margins with less extensive surgical extirpation. Rarely, Paget’s disease may become invasive, and metastases to groin nodes have been reported.86–94 Management including radiation has been reported with anecdotal favorable outcomes both in patients with invasive and noninvasive Paget’s disease.95–98 Radiation doses of 40 Gy to 56 Gy have been utilized in patients for whom the scope of surgery required to attempt surgical clearance would be medically unfeasible or functionally mutilating.
Clinical Presentation of Invasive Squamous Cancer of the Vulva
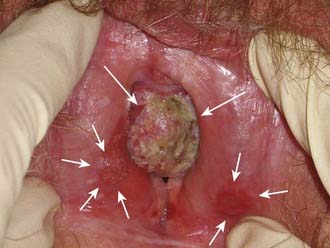
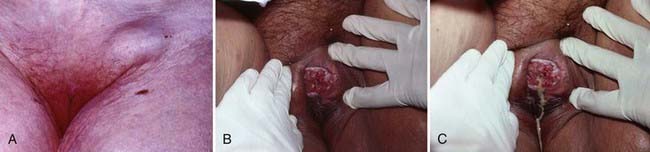
Pruritis or a localized sore are common presenting symptoms of vulvar cancer. Burning after urination may occur when urine comes in contact with areas of epithelial destruction and inflammation. Spotting and serous oozing may be noticed by the patient, but major hemorrhage is unusual. Most vulvar cancers arise in the labia, with only 15% involving the clitoris. The tumor is often exophytic, with a raised velvety red appearance, although centrally ulcerated lesions undermining surrounding skin may also be seen. Vulvar cancer may coexist with, and mimic, the gross appearance of venereal warts (condyloma acuminatum), which may result in neglect by the patient or delay in diagnosis (Fig. 52-10). Many vulvar cancers occur in older women who are not receiving regular gynecologic care or pelvic examinations. Because of inappropriate modesty, some patients will not report local symptoms to their physician. In circumstances where a tumor is not obvious, a patient may undergo unsuccessful treatment for a presumed dermatologic condition before a diagnostic biopsy. Because of patient and physician factors, delays in diagnosis of a year or longer after development of symptoms are not uncommon.
Routes of Spread
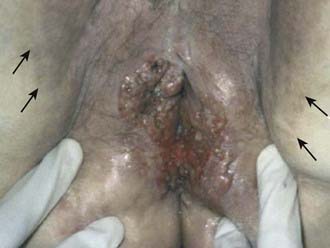
Vulvar cancer involves adjacent structures by direct extension. Invasion of the vagina is common (stage T3), often in conjunction with involvement of the distal urethra. When vaginal involvement is extensive, invasion of the bladder may be observed, or destruction of the rectovaginal septum with involvement of the rectal mucosa (stage T4). Extension to the perineum is frequent, often with involvement of anal or perianal skin. Extensive lesions may invade the anal sphincter. Satellite lesions in adjacent skin may occur, presumably by transit through dermal lymphatics. Squamous cancers of the vulva are autotransplantable, with “contact lesions” occupying mirror-image locations on the labia (Fig. 52-11). Rarely, extensive permeation of regional dermal lymphatics presents a picture analogous to peau d’orange of the skin of the breast (Fig. 52-12).
The earliest noncontiguous spread is to regional lymph nodes in the groin, which will be contaminated in approximately 30% of patients with operable vulvar cancer. Discrete (<2-cm diameter), well-lateralized primary cancers limited to the vulva and not approaching midline structures rarely spread to contralateral groin nodes in the absence of spread to ipsilateral nodes.23,24,99–104 Although direct lymphatic channels from the clitoris to the deep pelvic nodes have been described, these pathways are rarely of clinical significance.16,105 Direct lymphatic channels from the clitoris and anterior vulva to the femoral nodes exist, and probably account for occasional patients with metastatic spread to the deep femoral nodes without superficial inguinal node metastasis.74,83,101,106,107 Metastatic spread to pelvic nodes will be found in approximately 15% to 25% of patients with groin node metastasis, but only very rarely without previous gross involvement of the inguinal or femoral nodes and/or metastatic contamination of multiple groin nodes.6,7,17,18,108–115
Diagnostic Assessments
The joint 2010 staging system (Table 52-1) of the Fédération Internationale de Gynécologie et d’Obstétrique (FIGO), the American Joint Committee on Cancer (AJCC), and the Union Internationale Contre le Cancer (UICC) is a mixed clinical, surgical, and histopathologic staging system that relies on clinical examination and selected biopsies for the assignment of T stage and the results of biopsies or therapeutic node dissection for the assignment of N stage.
Table 52-1 AJCC 2010 Staging Classification of Cancer of the Vulva
Potentially useful assessments to define extent of disease before therapeutic intervention include careful pelvic examination (possibly with anesthesia), chest radiography, computed tomography (CT), positron-emission tomography (PET), or PET/CT to assess regional nodes, and soft tissue optimized pulse sequence magnetic resonance imaging (MRI) to assess regional soft tissue extensions. For patients with very extensive lesions, cystourethroscopy and/or proctosigmoidoscopy may be indicated. For patients with clinically apparent groin node metastases, CT or PET/CT may be used to assess possible spread to pelvic or upper retroperitoneal lymph nodes. Histologic confirmation of groin node metastasis by fine-needle aspiration (FNA) cytology or imaging guided core biopsy is desirable before planning therapy as approximately 22% of clinically suspicious groin nodes will be benign and enlarged consequent to reactive or inflammatory changes (Fig. 52-13).2,7,18,109,111,116 A patient who will be treated with radiation must undergo a pelvic CT scan both to screen for gross adenopathy that may be too deep to palpate and to conduct accurate dosimetric planning of radiotherapy directed to the inguino-femoral nodes.
Identification of sentinel lymph nodes by lymphoscintography or isosulfan blue intradermal injection or both is an area of active clinical investigation.74,117–130 Lymphatic mapping may be helpful for selection of patients with lateralized primary cancers in whom treatment of the ipsilateral groin may suffice. Depending on surgical philosophy and the threshold for use of adjunctive therapies, confirmation of sentinel lymph node metastasis may alter the extent of surgical therapy both for patients with squamous cancers of the vulva and those with melanoma. However, treatment algorithms based on sentinel node identification and biopsy are not yet broadly accepted.
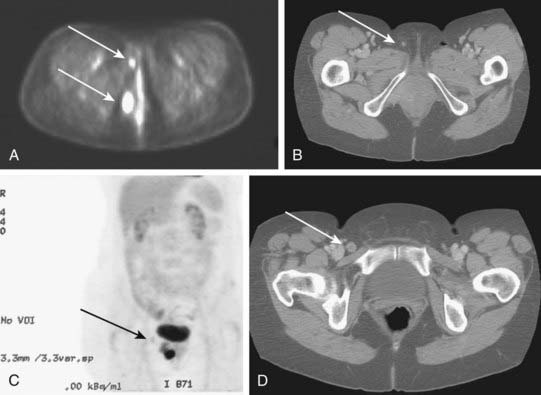
PET may be useful in evaluating patients with vulvar melanoma for distant metastases. A role for PET in squamous cancer has not been established. Preliminary evidence suggests relative insensitivity in detection of nodal metastases in the groins (67%), but a high specificity (95%).131 Thus, a negative groin PET alone should not exempt a patient from some form of therapy directed to the regional nodes. A positive study, particularly in a patient to be treated with definitive or preoperative radiation-based therapy, may assist in the selection of target volume and radiotherapy dose. Whenever possible, histologic confirmation of nodal status should be obtained by needle biopsy under ultrasound or CT guidance, or by excision. Fig. 52-14 illustrates the potential value of PET/CT in defining initial disease extent before treatment.
Standard Therapeutic Approaches
Surgery
For approximately 40 years, en bloc radical vulvectomy and bilateral regional lymphadenectomy (inguino-femoral with or without pelvic), using a single “butterfly” or trapezoidal incision, was the standard of care for patients with operable carcinoma of the vulva. The most common postoperative complication, frequently responsible for prolonged hospitalization and delayed recovery, is breakdown of the surgical wound in the groin. This complication is markedly reduced in frequency and severity by performance of the vulvectomy and the groin dissections through separate incisions, which serves to reduce tension on the skin flaps, preserve vascular supply, and improve the probability of primary healing.132,133 This refinement in technique, customarily reserved for patients with clinically uninvolved groins, comes at the price of the rare recurrence in the preserved bridge of tissue between the separate incisions. These failures may represent in-transit or retrograde lymphatic metastasis, unlikely events unless groin nodes are extensively contaminated.
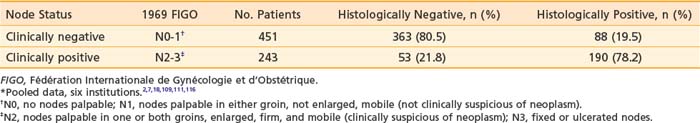
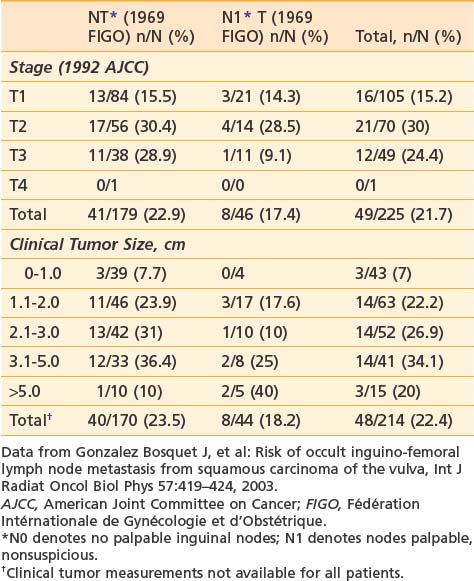
It is essential to appreciate that much of the classic surgical literature analyzes results using the 1969 staging system of FIGO, which employed clinical evaluation of both the primary tumor and the groin nodes. In 1989, FIGO adopted modifications to this staging system, resulting in a hybrid, clinicopathologic staging system that incorporated the results of histologic assessment of regional nodes, explicitly acknowledging the importance of nodal status in determining prognosis and therapy and implicitly recognizing the inaccuracies of clinical assessment of the regional lymph nodes by palpation. Approximately 20% of patients with clinically uninvolved groin nodes (1969 FIGO including N0 patients with nonpalpable groin nodes or N1 patients with groin nodes palpable, but not enlarged or suspicious) have histologic evidence of groin metastasis if subjected to radical groin dissection.* Approximately 22% of patients with clinically suspicious groin nodes (1969 FIGO including N2 patients with enlarged, firm, mobile nodes considered clinically suspicious, and N3 patients with fixed or ulcerated nodes) show no histologic evidence of nodal spread if the groin nodes are dissected (Table 52-2).*
The prognosis of surgically treated vulvar cancer is directly related to the presence or absence of regional node metastasis,134 the extent of lymph node infestation, and the anatomic level of nodal involvement. Five-year disease-free survival probability is approximately 95% for patients with T1-2N0 tumors7,135,136 decreasing to approximately 70% for stage T3N0.7 Disease-specific survival drops to approximately 64% at 5 years when groin nodes are involved at the time of initial treatment.134 Most recurrences are detected within 2 years of initial therapy. The probability of recurrence within 2 years is approximately 5% for surgically treated node negative patients, and approximately 30% for surgically treated patients with node metastases.134,136 Metastasis to a single node is prognostically less ominous than involvement of multiple nodes.16,17,137 Patients with unilateral node metastasis fare better than those with bilateral spread116 and 5-year disease-free survival drops to approximately 40% when multiple nodes are involved. Approximately 20% of patients with pelvic (iliac) node metastasis can be cured by locoregional therapies (surgery, radiation, or combined modality therapy).*
Tumor size, depth of invasion, presence of vascular or lymphatic space invasion, pattern of tumor growth and invasion, and histologic grade seem to be important predictors of lymph node spread as well as prognosis, although there is no consensus concerning the relative importance of each parameter in multivariate analysis.† Surgical specimens from patients with primary tumors 2 cm or less in diameter (T1) clearly show escalating risk of node metastasis with progressive depth of invasion (Table 52-3).‡ The risk of clinically occult involvement of inguino-femoral nodes in patients with palpably nonsuspicious groins undergoing complete groin dissection is tabulated by primary tumor size and T category in Table 52-4.141
Table 52-3 Incidence of Groin Node Metastasis Correlated With Depth of Invasion for Primary Tumors <2 cm*
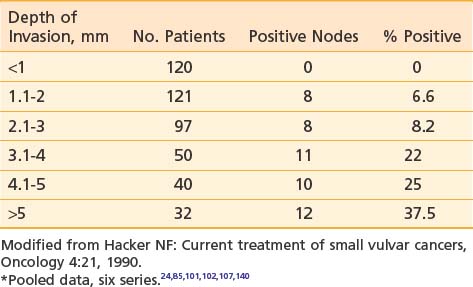
Table 52-4 Probability of Clinically Occult Metastasis to Inguino-Femoral Lymph Nodes Correlated With Primary Tumor Category and Size
An infiltrative pattern of growth correlates with nodal spread, and the presence of vascular or lymphatic space invasion substantially escalates the probability of finding metastases in dissected nodes.104,115,142 The presence of metastatic spread to contralateral groin nodes in the absence of disease in ipsilateral nodes occurs in 15% or less of all patients with metastases to groin nodes,112 generally in patients with larger lesions. Although described in two patients with lateralized T1 lesions,50 the risk of contralateral nodal spread in the absence of ipsilateral metastasis is less than 3%.§ When metastatic disease is present in multiple groin nodes, pelvic lymphadenectomy will detect disease in 15% to 25% of patients.6,7,17,18,108–115 but rarely when only one groin node is microscopically contaminated.16,17 A thoughtful appraisal of the risk of nodal spread and the anatomical level of potential contamination is an essential part of determining target volume, dose, and technique for a course of radiation therapy, whether the radiation is given preoperatively, postoperatively, or as primary treatment.
Integration of radiation and surgery for optimal outcomes requires an understanding of the inherent strengths and limitations of primary surgical treatment and the extent of operative therapy to be performed. Surgical strategy is predicated on primary tumor parameters, clinical assessment of the groin nodes, the status of adjacent tissues, patient age and vigor, and patient preference. Based on an appreciation by the surgeon of clinicopathologic risk factors and an ability to identify patients who have minimal risk of nodal spread, radical wide local excision (employing a clinical margin of at least 1 cm in all dimensions) without node dissection has been reported to be a sensible surgical option for select patients with discrete tumors 2 cm or less in diameter, with 1 mm or less of invasion, and without an infiltrating or “spray” pattern of growth. Radical wide local incision implies a significantly deeper excision than is routinely employed for VIN. Radical excision extends to the level of the deep perineal fascia usually 1 to 2 cm below the skin. Provided the surgical margins are adequate, local recurrence will be observed in only 5% of such patients.22,24,101,103 Radical wide local excision may be appropriate for patients with larger or more deeply invasive primary tumors, but it should be accompanied by at least ipsilateral groin dissection. For patients with very well-lateralized T1 and T2 primary tumors confined to the labia, contralateral groin dissection may be omitted unless there is spread to the ipsilateral groin.22–24,99–104,143 Unless conservation of the clitoris and sexual function are of major importance to the patient, radical vulvectomy will generally be the most appropriate surgical management of the primary tumor for patients with multifocal invasive vulvar cancer, or patients with invasive cancer associated with extensive VIN or vulvar dystrophy that has been unresponsive to topical steroids.
The best clinical predictor of local recurrence is the adequacy of the surgical margin (Table 52-5).144–146 An 8-mm margin in fixed tissue, corresponding to a clinical margin of 1 to 1.5 cm, is a useful guide in planning conservative surgery for patients with primary tumors that approach or extend to critical normal structures such as the anus, urethra, distal vagina, and clitoris. The distal urethra (no more than 1.5 cm) can be removed with preservation of urinary continence in most patients147 although removal of only a portion of the anal sphincter may compromise fecal continence.148 The risks of both local recurrence and loss of normal tissue function must be judiciously assessed and compared with risks associated with alternative strategies, such as those employing preoperative radiation or chemoradiation with less extensive surgery or chemoradiation alone. Exenterative surgery, even for massive primary disease, should seldom be chosen as initial therapy unless the cancer has already destroyed the functions of the critical normal tissues that might otherwise be preserved. It is rarely appropriate when regional node metastases are present.8
Table 52-5 Width of Surgical Margin and Risk of Local Recurrence
| Series | Margin <8 mm, n/N (%) | Margin ≥8 mm, n/N |
|---|---|---|
| Heaps146 | 21/44 (48%) | 0/91 |
| Chan145 | 12/53 (23%) | 0/30 |
| De Hullu144* | 9/40 (23%) | 0/39 |
* Surgical margin of <8 mm versus >8 mm.
The extent of groin dissection in the setting of confirmed metastasis to one or more groin nodes remains controversial. Outcomes analysis comparing nonrandomized patients undergoing complete groin dissection followed by radiation versus resection of enlarged/suspicious nodes only followed by radiation suggest that the limited surgical approach is associated with equivalent control in the groin (groin relapse-free survival) and no change in overall survival. Theoretically, both acute and late morbidities could be expected to be less among patients undergoing less extensive groin dissection before radiation, but complications and quality of life could not feasibly be assessed in this multi-institutional study.149
Chronic leg lymphedema may develop in 30% or more of patients undergoing radical dissection of superficial and deep inguino-femoral nodes. In an effort to reduce this risk, the concept of sentinel node dissection has been extended to the management of vulvar cancer during the past decade.74,117–130 Among patients at risk for groin node metastases, negative sentinel nodes may identify those least likely to benefit from radical dissection of groin nodes. Gynecologic Oncology Group protocol 173 is an active trial of sentinel node mapping in patients with vulvar cancer. In this study, sentinel nodes are identified by blue dye and/or lymphoscintography. Subsequent to surgical excision of the sentinel nodes, a comprehensive groin dissection is performed. The principle endpoints of the study are to quantify the negative predictive value of a sentinel node biopsy and to describe the anatomical location of sentinel nodes. This protocol is intended to accrue a total of 630 patients.
A multi-institutional study from The Netherlands reported preliminary outcomes for 403 patients with T1/T2 cancers of the vulva undergoing sentinel node procedures studying a total of 623 groins. When the sentinel nodes were found to be histopathologically negative, inguino-femoral lymphadenectomy was omitted. With a median follow-up of 35 months, 6 groin recurrences (2.3%) were diagnosed among 259 patients with a negative sentinel node and unifocal vulvar disease, and 3-year survival was estimated to be 97%. Chronic complications were markedly reduced in patients undergoing groin surgery limited to the sentinel nodes compared to patients undergoing sentinel node removal followed by inguino-femoral lymphadenectomy. Recurrent episodes of erysipelas were seen in 0.4% of the former compared to 16.2% of the latter. Leg lymphedema was reduced from 25.2% in patients undergoing complete groin dissection to 1.9% in patients undergoing only sentinel node removal.117 This study would suggest that the negative predictive value of a sentinel node study is high. However, as the consequence of groin relapse is usually a fatal outcome in patients with vulvar cancer,110,150–155 it will be critical to know the false negative percentage with the greatest possible precision before abandoning traditional surgical therapy associated with an excellent probability of regional cancer control.
Approximately 80% of patients in whom surgical therapy fails suffer recurrences in the residual vulva, on the perineum, at the vaginal introital margin, in the groins or upper thighs, or in the pelvis, and less than 20% have recurrences distantly.24,25,150–152,156–158 Parameters that are predictive of locoregional recurrence include the width of the surgical margin, the presence of groin node metastasis, vascular or lymphatic space invasion, and an infiltrative pattern of growth. The presence of one or more of these factors should prompt consideration of adjuvant radiation.
Radiation Therapy
Radiotherapy is increasingly employed in the curative management of vulvar cancer, often in conjunction with synchronous administration of radiation potentiating chemotherapy. Radiation salvages some patients with locoregional recurrence after radical vulvectomy and regional node dissection.155 Postoperative radiation directed to the groins and pelvic nodes improves disease-free survival in patients with metastatic spread to two or more inguino-femoral nodes25,156 and may further improve results if residual vulva and perineum are included within the irradiated volume.159 Preoperative radiation or chemoradiation have reduced the indications for exenterative surgery and may permit a substantial decrease in the volume of normal tissue that must be removed in patients with tumors invading or intimately approximating functionally important midline structures (anus, clitoris, urethra, and vagina).* Radical chemoradiation provides sensible alternative therapy for patients who are technically unresectable or medically inoperable.36,40–43
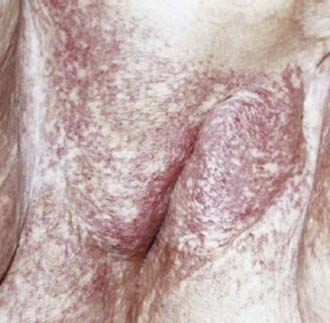
 ). Planning did not compensate for lesser patient anterior to posterior dimentions and beam attenuation at the level of the vulva and perineum. Estimated true dose approximately 10% greater with each fraction.
). Planning did not compensate for lesser patient anterior to posterior dimentions and beam attenuation at the level of the vulva and perineum. Estimated true dose approximately 10% greater with each fraction.