34 Cancer of the Thyroid
Epidemiology
Primary thyroid cancer is the most common endocrine malignancy, with an estimated incidence of 37,340 cases in the United States per year.1 This incidence is increasing, and thyroid cancer is now the eighth most common malignancy in women and the second most common malignancy in women under 40 years of age.2 Most, but not all, of this increase is a consequence of the increased detection of smaller WDTC lesions.2 Autopsy studies suggest that there is a large number of people in the general population with small, subclinical WDTC lesions.3
With regards to gender, women represent 76% of newly diagnosed cases.1 With regards to race, the incidence per 100,000 Caucasian, Asian, Hispanic, and Black women, is 4.9, 4.1, 3.5, and 2.7, respectively.4 In the San Francisco Bay area, well-controlled epidemiological studies indicate that the rate of thyroid cancer is higher in women of Southeast Asian descent.5,6
Environmental and dietary factors may also impact the frequency and distribution of thyroid cancer subtypes in a population. Ionizing radiation,7 goitrogens,8 and familial syndromes,9 have all been associated with PTC. People exposed to ionizing radiation during the atomic exposures at Hiroshima and Nagasaki in 194510 and children living near the Chernobyl nuclear accident in 198611 have a higher incidence of PTC. Furthermore, external beam radiation treatments in children for conditions such as acne, ringworm, birthmarks, enlarged thymus glands, or tonsillitis are associated with a higher risk of PTC later in life.12 Iatrogenic radioiodine exposure also slightly increases the risk of developing thyroid cancer and benign thyroid disorders.13 The incidence of FTC is higher in iodine-deficient regions, while PTC rates are higher in iodine-replete regions.14 Familial PTC has been described in numerous families, although the gene or genes responsible for this syndrome has not been elucidated.15 With regards to prognosis, patients with familial PTC with more than two members with PTC have a worse prognosis than those with sporadic disease.16
ATC accounts for more than 50% of the thyroid cancer deaths in the United States, despite the fact that it represents less than 2% of all thyroid cancers diagnosed.17 A higher incidence of ATC is seen in areas with low socioeconomic status and a high incidence of endemic goiter.18 There is ample evidence that ATC originates through the dedifferentiation of WDTC cells; thus, incomplete or delayed treatment of WDTC increases the risk of anaplastic transformation.19
Molecular Basis of Thyroid Cancer
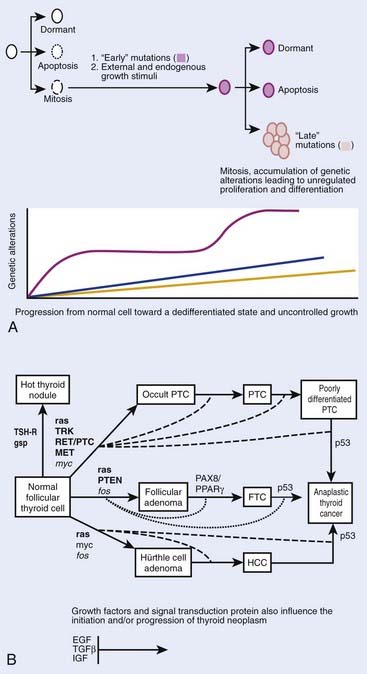
Although thyroid oncogenesis is not completely understood, some of the genetic changes that drive malignant transformation have been described for each histological subtype of thyroid cancer (Fig. 34-1). Thyroid oncogenesis entails the stepwise progression of molecular alterations that dysregulate cellular proliferation, apoptosis, and invasion.
Most sporadic PTCs harbor an activating mutation of receptor tyrosine kinases or the RAS-RAF-MEK-ERK pathway.20 These mutations are generally nonoverlapping, suggesting that such activation plays a central role in thyroid oncogenesis.20 The B type RAF kinase (BRAF) is the most prominent RAF kinase in thyrocytes, and point mutations in BRAF are found in approximately 45% of sporadic PTC.21 The presence of BRAF mutations in PTC microcarcinomas suggests that it plays a role in the initiation of thyroid oncogenesis.22 The BRAF V600E point mutation is reportedly associated with aggressive tumor behavior. PTCs with this mutation have higher rates of lymph node metastases and local invasion, resulting in higher rates of recurrent and persistent disease after thyroidectomy.23 Lastly, intra- and interchromosomal inversions of the RET proto-oncogene (RET/PTC) can also be found in PTC, which results in constitutive activation.24 These RET/PTC rearrangements are seen in approximately 75% of PTCs associated with childhood exposure to ionizing radiation.25,26
Approximately 25% of all MTC cases are inherited in an autosomal dominant fashion in one of three familial syndromes.27 In MEN2A, MTC is associated with pheochromocytoma and hyperparathyroidism.28 In MEN 2B, MTC is associated with pheochromocytoma but not with hyperparathyroidism, and patients are characterized by a Marfanoid appearance and oral mucosal neuromas.28 Lastly, isolated familial MTC is marked by the autosomal dominant transmittance of MTC with no other associated endocrinopathy.28
The confirmation that MTC could be part of a familial syndrome prompted a considerable effort to uncover its molecular basis, which was later identified as a point mutation in the RET proto-oncogene.29 The RET proto-oncogene is a tyrosine kinase receptor located on chromosome 10. Interestingly, somatic mutations in the RET proto-oncogene also account for approximately 25% to 30% of sporadic cases of MTC.30 MTC tumor behavior and clinical outcomes are heterogeneous, and prior studies have demonstrated that tumor behavior is contingent upon genotype. For example, the somatic 918 RET mutation is associated with aggressive MTC behavior. These important molecular discoveries have shifted the focus of MTC to early identification and prophylactic surgery, which have improved patient outcomes.31,32
Although virtually all ATC have a p53-related defect, the mechanisms of anaplastic transformation remain incompletely understood.33,34 ATC is likely derived from the dedifferentiation of WDTC and studies suggest that activation of both RAS-RAF-MAPK and PI3K pathways may play a role in anaplastic transformation.35,36 One study found an increasing frequency of genetic alterations in RAS-RAF-MAPK and PI3K/Akt in ATC relative to DTC when these histological subtypes coexisted in the same specimen.37 In the same study, they found that the only overlapping mutations in matched WDTC and ATC were in BRAF and PIK3CA.37 Conversely, a different study showed that BRAF and p53 were both altered in ATC, but that they were not overlapping with RAS or RET/PTC mutations.38
Anatomic Considerations
The thyroid gland wraps around the anterolateral trachea with two shield-like lobes connected by an isthmus, giving an appearance that is vaguely similar to a butterfly (Fig. 34-2). The most useful surface anatomy landmark is the cricoid cartilage, which is found just cephalad to the thyroid isthmus. The cricoid cartilage is palpable caudal to the tracheal cartilage, or Adam’s apple. Alternatively, the cricoid cartilage can be found by identifying the sternal notch and palpating the trachea in a cephalad direction; the cricoid cartilage will usually be the first prominent cartilage palpated using this technique. The pyramidal lobe, which is the distal remnant of the thyroglossal duct, is a narrow piece of thyroid tissue that extends cranially from the isthmus. An unresected pyramidal lobe is occasionally the cause of remnant thyroid tissue detected on postoperative RAI scans. Aberrant thyroid tissue can be found in the midline, and very rarely, lateral or inferior to the main body of the thyroid gland within the central neck. More commonly, thyroid cells discovered from FNA samples lateral to the carotid sheath and thyroid gland represent metastatic WDTC.
The lymphatic drainage of the thyroid gland flows in all directions and is inconsistent. The anatomic classification scheme that defines the cervical lymphatic subdivisions was most recently updated by the Committee for Head and Neck Surgery and the American Academy of Otolaryngology-Head and Neck Surgery (Fig. 34-3).39 The central neck compartment, designated level VI, is located between the carotid sheaths. The lateral neck compartments, including the jugular chain (level II to IV) and posterior neck (level V), are generally secondary drainage basins after the central neck.
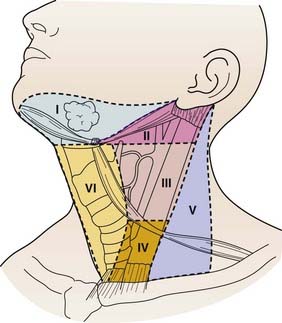
FIGURE 34-3 • Lymph node anatomic classification: the six levels of cervical lymph nodes in the neck.
(From Robbins KT, Clayman G, Levine PA, et al: Neck dissection classification update; Revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology—Head and Neck Surgery, Archives of Otolaryngol Head Neck Surg 128:751–758, 2002, p 752.)
The pattern of metastases in PTC typically begins with spread to ipsilateral central and then ipsilateral lateral lymph nodes, and then later to contralateral cervical lymph nodes.40 However, reports of “skip” metastasis to the lateral neck with normal central neck lymph nodes have been reported in about 20% of patients with PTC and MTC and may be more common in patients with primary tumors located in the superior thyroid poles. Perithyroidal, pretracheal lymph nodes, termed “Delphian” lymph nodes, when found to harbor metastatic disease, are associated with higher incidence of central and lateral compartment metastases.41 PTC can also spread to distant sites, including the lungs and bones in approximately 4% of newly diagnosed patients.42
Lymph node metastases are found in less than 6% of FTC in contrast to 6% to 30% of patients with HCC.43 Lung metastases from hematogenous spread are more common in FTC, HCC, and ATC. Liver metastases are relatively common in MTC, but are rare in thyroid cancers of follicular cell origin.
Physiology
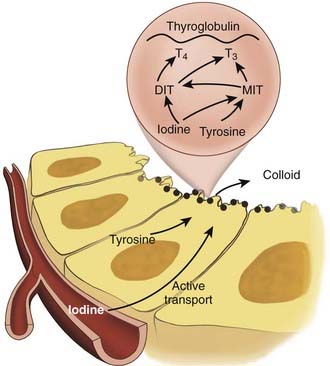
The principal endocrine function of the thyroid gland is the production and controlled release of thyroid hormone and calcitonin. Systemic thyroid hormone levels impact development, growth and metabolism. The two forms of thyroid hormone, triiodothyronine (T3) and thyroxine (T4) are synthesized within thyroid follicles. Thyroglobulin is a large glycoprotein synthesized by follicular cells, which is the source of tyrosyl residues needed for thyroid hormone synthesis. Both T3 and T4 are synthesized through the coupling of iodide with tyrosyl residues in a reaction involving the enzyme thyroperoxidase (Fig. 34-4) Newly synthesized thyroid hormone is bound to thyroglobulin protein and stored in colloid, which can later be released into the systemic circulation. Hydrolysis of thyroglobulin protein and release of thyroid hormone is regulated by TSH levels and the availability of iodide.
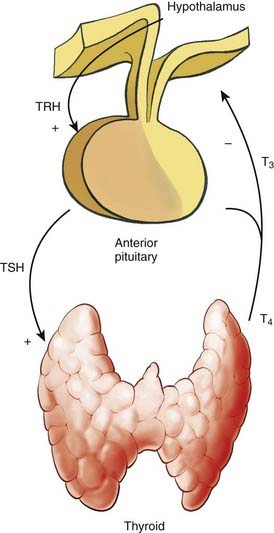
Thyroid hormone levels are tightly regulated in a negative-feedback manner within the hypothalamic-pituitary-thyroid axis (Fig. 34-5). Thyroid stimulating hormone (TSH) is the central element in this axis, which directly controls the activity and proliferation of follicular cells. TSH is synthesized and secreted by the anterior pituitary, a process that is regulated by thyrotropin-releasing hormone and blood thyroid hormone levels. Thyrotropin-releasing hormone is synthesized in the hypothalamus and is transported to the pituitary via the hypophyseal portal system, a process that is suppressed when systemic T3 levels are high.
The TSH receptor is a G-protein-coupled receptor which upregulates thyroid hormone production and release via cAMP associated signaling. Because TSH can induce the proliferation of both normal thyrocytes and WDTC of follicular origin, suppression of TSH is an important feature of thyroid cancer treatment. Furthermore, TSH receptor mutations44 and a higher concentration of TSH receptors45 have been associated with autonomous follicular thyroid neoplasms.
Clinical Presentation and Diagnosis
In addition to a complete history and physical exam, patients that present with a neck mass suspicious for a thyroid neoplasm should undergo formal neck ultrasound by an experienced ultrasonographer to characterize the lesion, monitor the remainder of the thyroid gland for abnormalities, and to evaluate for abnormal cervical lymph nodes. FNA biopsy is a reliable diagnostic tool that should be performed on thyroid lesions with suspicious sonographic characteristics, such as internal microcalcifications. FNA has a sensitivity of 95% to 98% and specificity of 97% to 99%, when performed by an experienced cytopathologist.46 Unfortunately, FNA is not an effective for discriminating FTC from benign follicular adenomas, or HCC from Hürthle cell adenomas. Histologic architecture is required to determine if a follicular or Hürthle cell neoplasms are malignant; capsular or vascular invasion must be seen to diagnose carcinoma in these subtypes. The current standard of care in the evaluation of a follicular neoplasm requires diagnostic hemithyroidectomy with formal histological review to determine if the lesion has invasive features consistent with carcinoma. Molecular-based tools will likely eliminate the need to perform diagnostic surgery for such patients; several groups have recently used tumor gene expression to define molecular signatures that can discriminate benign from most malignant follicular tumors.47–49
Patients with sporadic MTC typically present with solitary thyroid nodules situated at the upper lateral regions of the thyroid gland, where the preponderance of C cells are located. On physical exam, MTC are described as hard, nodular tumors that are sometimes calcified. Patients with locally invasive MTC often complain of “aching” or painful thyroid tumors. Carcinoembryonic antigen (CEA) levels are almost always elevated in patients with MTC, which is the same serum protein marker utilized to screen colorectal cancer patients for recurrence after resection. Since MTC can secrete several proteins (e.g., somatostatin, vasoactive intestinal peptide, adrenocorticotropic hormone (ACTH), bombesin), patients with advanced disease and hepatic metastases can present with generalized symptoms, such as flushing, Cushing’s syndrome, and diarrhea is common.50
The diagnosis of MTC is reliably established by FNA, which reveals amyloid and may stain positive for calcitonin, CEA, and chromogranin A.51 In relatives of patients with familial MTC, small tumors and C-cell hyperplasia can be diagnosed by elevated basal or stimulated levels of calcitonin. High CEA levels appear to be a worse prognostic indicator than elevated blood calcitonin levels.
In comparison with sporadic MTC, hereditary MTC generally occurs in younger patients and can be seen in the context of the isolated familial MTC and MEN2 syndromes. A recent claim that former American president Abraham Lincoln may have had MEN2B seems unlikely,52 given that untreated patients with this syndrome typically die within the first 3 decades of life, with few reports of asymptomatic long-term survival.53 Although there are some RET proto-oncogene mutations that result in MTC in older patients, patients with MTC and MEN2B have the worst prognosis of all patients with MTC.
Patients with ATC typically present with a rapidly enlarging neck mass that is fixed to surrounding structures. ATC are generally large and cause local obstructive symptoms from their mass effect, including pressure in the neck, dysphagia, and dysphonia. Patients with thyroid lymphoma can also present in a similar fashion. In our own experience with ATC, the average tumor size was 7 cm and the majority of patients presented with symptomatic disease. Furthermore, invasive thyroid tumors can invade the recurrent laryngeal nerves and cause vocal cord dysfunction and regional pain. ATC can be readily confirmed by FNA biopsy with cytological review.54 Distant metastases are common at presentation and can be found in up to 43% of patients.55 Both distant and regional metastases essentially preclude curative therapy for patients with ATC.
Prognostic Models and Staging of Thyroid Cancer
While the majority of patients with WDTC have excellent outcomes after surgical resection, some patients harbor aggressive disease. In an effort to select high-risk patients for adjuvant therapy, prognostic models utilize clinical and pathologic factors to predict survival (Table 34-1). Multiple large retrospective reviews have confirmed that age, tumor grade, distant metastases, tumor invasion, and tumor size are independent predictors of outcome after thyroidectomy.
The American Joint Committee on Cancer TNM system characterizes the anatomic status of the primary tumor, regional lymph nodes and distant metastases (Table 34-2). This system was updated in 2003, and is often applied to both WDTC and MTC. TNM staging can be used as a prognostic tool and also for research purposes to enable consistent stratification of patients.
Surgery for WDTC
Surgical resection is the primary treatment for patients with WDTC.56 Thyroidectomy is both safe and effective when performed by an experienced surgeon, with 10-year survival rates approximating 93% and 85% for PTC and FTC, respectively.17 Although most clinicians agree that total thyroidectomy is the primary treatment for patients with high-risk PTC, there is some controversy regarding the extent of surgery for patients with low-risk WDTC.57 Some clinicians argue that thyroid lobectomy is equivalent to total thyroidectomy, and avoids the potential morbidity of bilateral operation.58
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree