46 Cancer of the Testis
From a radiation oncologist’s point of view, when evaluating tumors of the testis one must first distinguish between seminomas, in which radiotherapy plays a major role in the care of the patient,1 and nonseminoma germ cell tumors (NSGCTs), in which the role of radiotherapy is primarily palliation and local control. This chapter focuses mainly on seminomas.
Incidence and Epidemiology
Testicular cancer accounted for some 8090 new cases and 380 estimated deaths in 2008.2 Although it represents only 1% of cancers in men it is the most common malignancy among men aged 20 to 30 years. Bilateral involvement is seen in 2% to 4% of patients. These tumors are highly curable even in advanced stage. The peak age incidence for seminoma is slightly older compared to NSGCT, with the histologic variant spermatocytic seminoma occurring in men older than age 60.3 Interestingly the worldwide incidence of testicular cancers has doubled in the past 40 years.2 Although germ cell tumors can rarely occur in extragonadal sites, their management follows that of testicular germ cell tumors.
Cryptorchidism, or an undescended testis, is the most studied risk factor for developing testis cancer. The relative risk of developing testicular cancer is 3.7 to 7.5.4,5 Orchiopexy before puberty decreases this risk.6 HIV as a risk factor is controversial. Although many reports show an increase in incidence,7 population-based studies have failed to confirm this.8,9 Chromosomal abnormalities most commonly involve chromosome 1 and 12. Deletions are seen in both arms of chromosome 1. The most frequent, and sometimes used diagnostically, is the isochrome of the short arm of chromosome 12, denoted as i(12p), and has been reported in up to 90% of germ cell tumors.10 Testicular cancers are observed 5 times more frequently among White men than among Black men. An association with the dysplastic nevus syndrome and Klinefelter’s syndrome has also been noted.
Anatomy
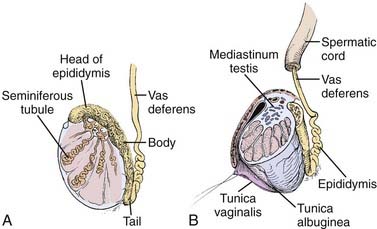
The adult testicle typically measures 4 to 5 cm in length and 2.5 cm in diameter. The testicle is covered by a parietal and visceral layer of the tunica vaginalis (Fig. 46-1). When this potential space accumulates fluid, a hydrocele forms. The testicle is encased by a hard outer capsule, the tunica albuginea. On the posterior-lateral aspect of the testicle, the tunica albuginea projects inwards, creating the mediastinum testis, the point of entry of blood vessels and ducts transporting sperm. Within the testicle, approximately 300 lobules exist, containing one or more seminiferous tubules: the site of spermatogenesis as well as site of origin for germ cell tumors. Each lobule of seminiferous tubules has short straight tubes that enter the mediastinum testis which then coalesce, forming the “rete testis” which, in turn, become fewer in number and wider in caliber, forming “efferent ductules.” These exit the testis and enter the head of the epididymis and subsequently the body and tail, where they become the vas deferens. Leydig cells reside in the interstitial tissue between seminiferous tubules and are responsible for testosterone production.
Pathology
The majority of testicular tumors are of germ cell origin. From a therapeutic perspective, these histologies are divided into two types: seminoma and NSGCTs. The histologies are listed in Table 46-1. The frequency of germ cell tumor histologies encountered is given in parentheses.
Table 46-1 Histologies of Testicular Tumors
| Germ Cell Tumors (95%) |
| Pure seminoma (40%) |
| Nonseminoma germ cell tumors (60%) |
| Non–Germ Cell Tumors (5%) |
| Stromal |
| Lymphoma |
Seminoma (35%)
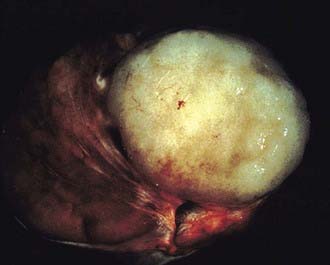
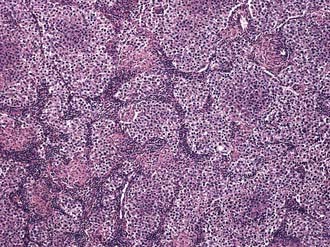
Three histologic subtypes of pure seminoma have been described. However, stage for stage, there is no prognostic significance to any of these subtypes. Classic seminoma accounts for 85% of all seminomas and is most common in the fourth decade of life. Grossly, coalescing gray nodules are observed (Fig. 46-2). Microscopically, monotonous sheets of large cells with clear cytoplasm and densely staining nuclei are seen (Fig. 46-3). It is noteworthy that syncytiotrophoblastic elements are seen in approximately 10% to 15% of cases, an incidence that corresponds approximately to the incidence of human chorionic gonadotropin (hCG) production in seminomas.
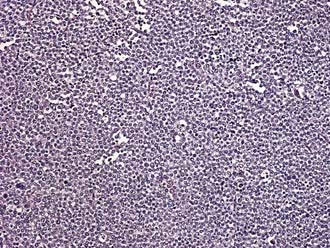
Spermatocytic seminoma accounts for 5% to 10% of all seminomas. Microscopically, cells vary in size and are characterized by densely staining cytoplasm and round nuclei that contain condensed chromatin (Fig. 46-4). More than half the patients with spermatocytic seminoma are over the age of 50. The important clinical aspect of spermatocytic seminoma is that they do not metastasize, thus, orchiectomy is curative and no additional therapy is needed.
Carcinoma in situ
A series of 250 patients with unilateral testicular cancer11 demonstrated the presence of carcinoma in situ (CIS) in 13 (5.2%) of the contralateral testes. This is approximately twice the overall incidence of bilateral testicular cancer. The presence of contralateral atrophy or ultrasonographic microlithiasis in patients with testicular tumors warrants contralateral biopsy. If diagnosed, CIS (also called testicular intraepithelial neoplasia, TIN) is usually treated by external beam radiotherapy to the involved testis with doses in the range of 18 to 20 Gy.12,13
Clinical Presentation
The classic presentation is that of a painless mass in the testes. Among the differential diagnoses are epididymitis, orchitis, and hydrocele. Trauma may lead to the discovery of an existing tumor but is not thought to be causative. Pain, though not common can be associated with presentation 40% of the time. Delay in diagnosis of greater than 3 to 6 months have been associated with worse prognosis.14 Evaluation of infertility occasionally leads to the diagnosis.15,16 Analysis of 38,000 men from the Surveillance, Epidemiology and End Results (SEER) database revealed that infertile men with abnormal semen analyses have a 20-fold greater incidence of testicular cancer compared to the general population.15 Gynecomastia and breast tenderness may be seen in 5% of patients17 owing to the production of estradiol in response to elevated levels of hCG. Patients with metastases to lymph nodes in the retroperitoneum most commonly present with low back pain and may or may not have hydronephrosis with secondary ureteral obstruction.
Serum Tumor Markers
Germ cell tumors frequently produce proteins that can serve as tumor markers for disease burden as well as diagnosis (Table 46-2). The two most important of these are AFP and the β subunit of human chorionic gonadotropin (β-hCG). Lactate dehydrogenase (LDH) level is also used as a marker for disease burden. It should be kept in mind, however, that these proteins are not specific to germ cell tumors. The serum half-life of these tumor markers is of clinical relevance and is 4 to 6 days for AFP; for β-hCG, 1 to 2 days. After 5 half-lives a previously elevated serum marker should return to within normal levels if all tumor cells have been eradicated. In pure seminoma elevated β-hCG is present about 10% of the time, yet if present tends to be modest in magnitude (usually <100). AFP, by contrast, is never present with pure seminoma.
Table 46-2 Frequency of Serum Marker Elevation in Testicular Tumors
| Histology | AFP, % | β-hCG, % |
|---|---|---|
| Seminoma | 0 | 9 |
| Teratoma | 38 | 25 |
| Embryonal | 70 | 60 |
| Choriocarcinoma | 0 | 100 |
| Yolk sac tumor | 75 | 25 |
AFP, Alpha-feto protein; hCG, human chorionic gonadotropin.
Routes of Spread
With the exception of choriocarcinoma, which demonstrates early hematogenous spread, germ cell tumors of the testis typically spread in a stepwise fashion via lymphatic channels. Lymph nodes of the testis extend from T11 to L4 but are concentrated at the level of the renal hila due to their common embryological origin with the kidney. The right and left testes possess somewhat different lymphatic pathways of clinical relevance in designing radiation fields. The primary landing site for the right testis is the interaortocaval area at the level of the right renal hilum. Stepwise spread, in order, is to the precaval, preaortic, paracaval, right common iliac, and right external iliac lymph nodes. The primary landing site for the left testis is the periaortic area at the level of the left renal hilum. Stepwise spread, in order, is to the preaortic, left common iliac, and left external iliac lymph nodes. In the absence of disease on the left side, no crossover metastases to the right side have ever been identified. However, right-to-left crossover metastases are common.18,19 These observations have resulted in modified surgical dissections to avoid injury to the sympathetic nervous system in select patients.
The retroperitoneum is the site most commonly involved in metastatic disease; however, visceral metastases may be seen in advanced disease. The sites involved in decreasing frequency include lung, liver, brain, bone, kidney, adrenal glands, gastrointestinal tract, and spleen.20 As mentioned, choriocarcinoma is the exception to the rule and is characterized by early hematogenous spread, especially to the lung. Choriocarcinoma also has a predilection for unusual sites of metastasis, such as the spleen.
Diagnostic Work-Up and Staging Studies
A testicular mass should be considered a tumor until proven otherwise. Work-up of a patient with a testicular mass should include an ultrasound of the both testes and tests for tumor markers, including AFP, β-hCG, and LDH. Once the diagnosis of testicular cancer has been pathologically established, one should then obtain a computed tomography (CT) scan of the abdomen and pelvis and a chest x-ray. Post-op serum markers should also be obtained, after an appropriate time interval, if they were elevated before surgery. A CT scan of the chest may be helpful to establish a baseline. Lymphangiography, although having the advantage of being able to detect nodal architecture, is now rarely used. The role of a positron-emission tomography (PET) scan in staging is controversial at this time. Its current utility lies in evaluating masses postchemotherapy to detect viable cancer cells. However, the decision to perform surgical resection of the residual mass should not be based exclusively on a positive PET image, since false-positive results appear to be common.21
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree