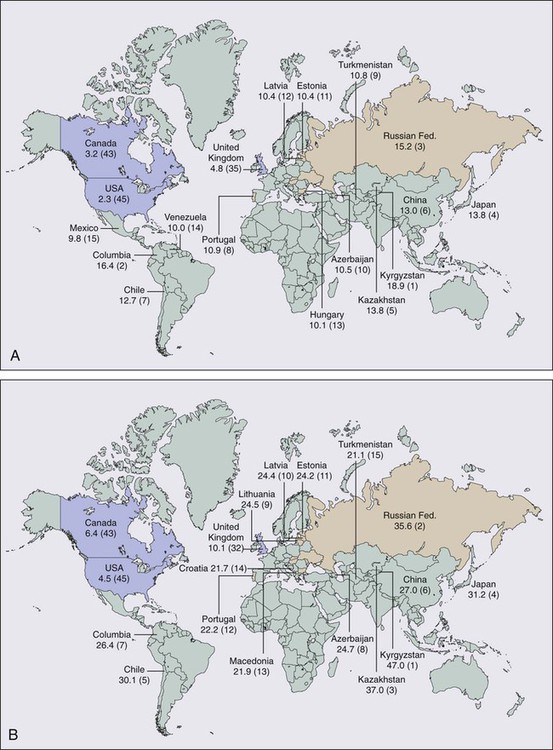
75 Leonard L. Gunderson, John H. Donohue, Steven R. Alberts, Jonathan B. Ashman and Dawn E. Jaroszewski • For stomach cancer in the United States, the expected incidence in 2012 was 21,320 cases and 10,540 deaths. • These cancers are usually adenocarcinomas. • In the United States, the site of origin is shifting as more proximal lesions are diagnosed. • Prognostic factors relate to tumor extent and include nodal involvement and extension beyond the gastric wall. • Ploidy may be an independent prognostic factor. • Staging should always include history and physical examination, complete blood cell count, liver chemistries, chest x-ray film, endoscopy with biopsy, ultrasound (determine degree of direct tumor extensions), and computed tomography (CT) of the abdomen (define extragastric disease). • Additional studies that may help define extent of disease include upper gastrointestinal imaging, CT of the chest (for gastroesophageal junction [GEJ] lesions), laparoscopy (to rule out peritoneal seeding or early liver metastases), and positron emission tomography. • Surgical resection is the primary therapy of resectable gastric and GEJ cancers. • Cure rates of 80% or higher are achieved only with early lesions (patients with nodes negative, confined to mucosa or submucosa), which are uncommon in the United States. • Role for extended node dissection has not been found in randomized trials. • Adjuvant therapy (chemotherapy, irradiation) is indicated on the basis of patterns of relapse and survival results with surgery alone (high rates of local-regional relapse and distant metastases). • Adjuvant chemotherapy has a modest, significant benefit and has become the standard in Asia. • Irradiation alone reduced local-regional relapse and improved overall survival (OS) in a Beijing trial of 370 patients testing preoperative irradiation versus surgery alone (5-year OS 30% vs. 20%, P = 0.009). • The U.S. intergroup phase III trial of 556 patients found a survival benefit for combined-modality postoperative irradiation plus chemotherapy versus surgery alone (3-year relapse-free survival 48% vs. 31%, P = 0.001; 3-year OS 50% vs. 41%, P = 0.005). • A British phase III trial of 503 patients demonstrated a survival advantage for perioperative ECF chemotherapy (epirubicin, cisplatin, 5-fluorouracil [5-FU]) when compared with surgery alone (5-year OS 36% vs. 23%, P = 0.009). • A French phase III trial of 224 patients demonstrated a survival advantage for perioperative cisplatin and 5-FU compared with surgery alone (5-year OS 38% vs. 24%, P = 0.02). • The POET trial of 120 patients with GEJ lesions tested preoperative chemotherapy versus chemoradiotherapy (CRT); outcomes trends favored preoperative CRT over chemotherapy alone for both OS (P = 0.07) and local control (P = 0.06). Locally Advanced Disease (Borderline Resectable/Unresectable) • Combined external beam radiation therapy (EBRT) plus chemotherapy or intraoperative radiation therapy (IORT) produced long-term survival in 10% to 20% of patients in most randomized and nonrandomized trials. • Neoadjuvant chemotherapy studies reveal possible increase in resection rates but high incidence of local-regional relapse (consider addition of IORT alone or with EBRT and concurrent chemotherapy to neoadjuvant chemotherapy regimens). • Palliative resection of gastric component of disease may be indicated. • European phase III trials demonstrate a trend toward improved quality and duration of life with palliative chemotherapy versus supportive care. Treatment of Metastatic Disease • Multiple-drug chemotherapy regimens have response rates of 30% to 50%, and provide some improvement in OS, including the two- and three-drug regimens ECF, EOX (epirubicin, oxaliplatin, capecitabine), and DCF (docetaxel, cisplatin, 5-FU). • A phase III trial of 594 patients showed a significant improvement in OS with the addition of trastuzumab to chemotherapy in patients with HER-2-positive tumors (median OS 13.8 vs. 11.1 months, P = 0.0046) In 2012, cancer of the stomach had an expected incidence in the United States of 21,320 cases and an expected number of 10,540 deaths.1 Age-adjusted gastric cancer death rates have decreased markedly in the United States since 1930 from approximately 28 to 2.3 in 100,000 women and from 38 to 5.2 in 100,000 men. Of the 45 countries in which age-adjusted death rates for gastric cancer were compared for 2000 (E-Fig. 75-1), the United States ranked 45th for both men and women.2 Kyrgyzstan ranked first for both men (47.0 in 100,000) and women (18.9 in 100,000). Factors that have been associated with a higher incidence of gastric cancer include smoked or salted foods, foods contaminated with aflatoxin, low intake of fruits and vegetables, low socioeconomic status, and possibly a decreased use of refrigeration.3,4 Possible occupational relationships include coal mining and rubber or asbestos workers. Precursor pathological conditions include pernicious anemia, achlorhydria atrophic gastritis, gastric ulcers, and adenomatous polyps. Between 5% and 10% of individuals with pernicious anemia subsequently develop malignancy. Prior partial gastrectomy for benign gastric or duodenal ulcer disease produces an increased risk of subsequent malignancy in the gastric remnant with latency periods of 20 years or more.5,6 Several studies have shown a threefold to sixfold increased risk of gastric cancer in individuals with Helicobacter pylori infection versus those with no infection, but the precise role of this bacterium in the etiology of gastric cancer remains unknown.9–9 A variety of bacterial, patient, and environment factors most likely act in combination to affect the development of gastric carcinoma. The increased association of H. pylori with gastric cancer seems to be mainly with distal gastric cancers and intestinal-type malignancy. Only a minority of H. pylori–infected individuals develop gastric cancer, and data do not yet exist on the effect of treatment of the H. pylori infection on subsequent malignancy. The most meaningful prognostic indicators relate to extent of tumor. With either hematogenous metastasis or peritoneal seeding, prognosis is almost always fatal. Recent immunohistochemical analysis of bone marrow aspirates has shown the presence of tumor cells to be an independent predictor of adverse outcome; however, confirmatory studies have yet to be published.10,11 Survival decreases with progressive direct tumor extension both within and beyond the gastric wall.12,13 Lymph node involvement, per se, is not as important as the number and location of nodes.16–16 Minimal lymph node involvement adjacent to the primary lesion results in the most favorable prognosis in node-positive patients, but even micrometastases in regional nodes may adversely impact survival.17 The solitary finding of either involved nodes or complete penetration of the gastric wall is usually not as ominous as the presence of both12,15 (E-Table 75-1). E-Table 75-1 Extent of Initial Disease Versus Survival Rates in Stomach Cancer* OS, Overall survival; DFS, disease-free survival. *Compilation of data from various series. The tumor grade and the gross and histologic pathological appearance of the primary malignancy seem to provide some prognostic information, but none of these factors is a prognostic variable independent of the tumor stage. Prognosis is generally worse with higher grade and diffuse-type carcinomas, which usually present with higher pathological stages of disease (see Fig. 75-1). Borrmann types I and II carcinomas have a relatively favorable 5-year survival rate, but patients with type IV tumors (linitis plastica) fare very poorly.18,19 Some investigators have suggested that tumors of the gastric cardia may have epidemiological factors different from cancers of the distal stomach20,21 and may exhibit different tumor biology.22 The prognosis is worse for cardia lesions,23,24 and flow cytometry reveals a greater incidence of aneuploidy when compared with tumors of the antrum and body.25 Flow cytometry provides valuable prognostic information for gastric cancer and may be an independent prognostic factor.25,26 As noted previously, aneuploidy is associated with unfavorable tumor location such as the cardia25,27 but is also associated with lymph node metastasis26,27 and direct tumor extension.26 Unfavorable DNA flow cytometry characteristics seem to relate closely to an unfavorable prognosis.25,26 In one series in which multivariate analysis of DNA ploidy was analyzed with other known prognostic factors such as stage, age, and sex, DNA ploidy carried statistically significant independent prognostic information.26 The presence of several peptides including estrogen receptor,28 epidermal growth factor receptor,29 the c-erbb2 protein,30 and plasminogen activator inhibitor type 119 seems to affect prognosis adversely. The expression of epidermal growth factor receptor and high levels of epidermal growth factor correlate with a higher incidence of primary tumor infiltration, poor histologic differentiation, and linitis plastica. The pathophysiological relationship between these peptide receptors and poor patient prognosis is not clear. Gastric cancers with class II major histocompatibility complex antigen expression (human leukocyte antigen [HLA]-DR) have a better prognosis, but the loss of expression is not an independent prognostic factor.31 Loss of tumor suppressor gene function, especially inactivation of the p53 gene, plays a key role in tumor suppression and cell-cycle regulation.32 The p53 gene puts a brake on DNA replication and triggers programmed cell death in response to DNA damage.33 Loss of p53 function is associated with the development of gastric cancer, impacts the effectiveness of chemotherapy and irradiation,34,35 and predisposes cells to genetic instability. A second aberration affecting gastric epithelial cells is alterations in mismatch repair genes, including HMSH3 and HMLH1, which account for replication errors throughout the genome. Mutations in these genes generate genetic instability and are associated with an increased tendency for the development of colorectal and gastric tumors.36,37 Two protooncogenes, c-met and K–sam, are associated with scirrhous carcinoma of the stomach. Overexpression of c-met correlates with tumor progression and metastasis, and K-sam encodes a tyrosine kinase receptor family.38 K–sam has a tendency to be activated in women with gastric cancer younger than 40 years of age and c-met to be amplified in men older than 50 years of age.39,40 Modern molecular biology observations confirm the heterogeneity of human gastric cancer.41 Gastric cancers with class II major histocompatibility complex antigen expression (HLA-DR) have a better prognosis, but the loss of expression is not an independent prognostic factor.42 Early detection would markedly improve the prognosis of gastric cancer in the United States, because surgical resection has a high cure rate with lesions limited to the mucosa or submucosa. However, the incidence of such early gastric cancers is less than 5% in most U.S. series. In Japan, the incidence of carcinomas confined to the mucosa or submucosa was only 3.8% in the 1955 to 1956 period. However, by 1966 the incidence of early lesions had increased to 34.5% because of vigorous screening procedures, leading to 5-year survival rates of 90.9% in this cohort of patients.43 Although mass screening has been useful in Japan to detect early cancers, defined high-risk populations have not existed in the United States in the past to justify the expense of widespread screening endeavors. Whether screening of individuals with H. pylori infection would be of value is not yet known. Individual practitioners should use upper gastrointestinal (GI) series or preferably endoscopy to screen patients who have occupational or precursor risk factors or individuals with persistent dyspepsia or gastroesophageal symptoms. Germline mutations in the CDH1 gene, which encodes the E-cadherin protein, have recently been recognized in families with hereditary diffuse gastric adenocarcinoma. Carriers of these mutations have a 70% lifetime risk of developing gastric cancer. Several reports of prophylactic gastrectomy have demonstrated the frequent presence of microscopic intraepithelial carcinomas in patients having regular endoscopic surveillance that includes multiple random biopsies.46–46 Early total gastrectomy has been recommended for this small patient population because of the lack of effective early tumor detection by less aggressive techniques. Microscopic evaluation of the proximal and distal resection margins for complete removal of the gastric mucosa is necessary, because residual gastric mucosa can degenerate and result in a gastric cancer.46 The terms gastric cancer and stomach cancer usually refer to adenocarcinoma, which accounts for 90% to 95% of all gastric malignancies. Other histologic types include lymphoma (usually intermediate- or high-grade histologic types), leiomyosarcoma, carcinoid, adenoacanthoma, and squamous cell carcinomas. The site of origin within the stomach has changed in frequency in the United States over recent decades, with more proximal lesions now being diagnosed and treated. The largest percentage of gastric cancers still arises within the antrum or distal stomach (around 40%), are least common in the body of the stomach (around 25%), and are of intermediate frequency in the fundus and esophagogastric junction (around 35%).47 Gastric carcinomas have been categorized by using both microscopic (Fig. 75-1) and gross pathological features. The Lauren classification system includes an intestinal type with improved prognosis that predominates in regions with high prevalence of gastric cancer, as well as a diffuse histologic type, with poor prognosis, which occurs more commonly in countries with low prevalence of stomach cancer.48 Grossly, gastric cancers can be categorized according to Borrmann’s49 five types: I, polypoid or fungating; II, ulcerating lesions surrounded by elevated borders; III, ulceration with invasion of the gastric wall; IV, diffusely infiltrating (linitis plastica); and V, unclassifiable. The Japanese Research Society for Gastric Cancer has a classification system that divides lesions into protruded (I); superficial (II) with elevated (IIa), flat (IIb), and depressed (IIc) subtypes; and excavated (III) types.50 Because of the numerous pathways of lymphatic drainage from the stomach, it is difficult to perform a complete nodal dissection (E-Fig. 75-2). Although initial drainage is usually to lymph nodes along the lesser and greater curvatures (perigastric or N1 nodes using the Japanese Research Society for Gastric Cancer designation), primary node drainage includes nodes along all three branches of the celiac axis (common hepatic, splenic, left gastric) and the celiac artery itself (Japanese N2 nodes).50 Node groups that are more distal include hepatoduodenal, peripancreatic, root of mesentery (N3), periaortic, and middle colic (N4). When proximal gastric lesions extend into the distal esophagus, the paraesophageal nodal system is at risk for involvement. The diagnosis of gastric cancer is usually confirmed by upper GI endoscopy, or radiographs (see diagnostic algorithm in Table 75-1). Double-contrast radiographs may reveal small lesions limited to the superficial (inner) layers of the gastric wall. Endoscopy is now the preferred initial diagnostic test, because it allows direct tumor visualization, cytologic testing, and direct biopsy for histology that yield the diagnosis in 90% or more of patients with exophytic lesions. Ulcerated cancers and linitis plastica lesions may be harder to diagnose endoscopically, but multiple biopsies and gastric washings for cytology enhance the probability of accurate diagnosis. Endoscopic ultrasound (EUS) has a high degree of accuracy in determining depth of tumor invasion (i.e., does the lesion extend beyond the muscularis propria?) but is less accurate in detecting regional nodal metastasis.53–53 Ultrasound-guided fine-needle aspiration for cytologic test allows the assessment of regional lymph nodes and some distant metastatic sites (e.g., liver), further enhancing the ability of EUS to determine tumor stage and resectability. Table 75-1 Diagnostic Algorithm—Gastric/Gastroesophageal Junction Cancer *Laboratory studies: CBC, creatinine, liver function studies (alkaline phosphatase, bilirubin, SGOT, LDH), albumin. With the development of laparoscopic general surgery, diagnostic laparoscopy is commonly used to assess for distant metastasis or unresectable locally advanced abdominal cancers. Several groups54,55 have reported the use of laparoscopy in stomach cancer patients. Metastatic disease was documented laparoscopically in 35% to 40% of patients.56–56 The sensitivity for metastases was 85% or greater55,56 and this technique was particularly sensitive in detecting liver and peritoneal disease. Laparoscopy is more sensitive and accurate in staging patients with regard to intraabdominal metastases than either ultrasound or CT scan.56,57 Many surgeons now routinely perform laparoscopy in all gastric cancer patients who are deemed candidates for surgical resection, to avoid nontherapeutic laparotomy. The current TNM (tumor, lymph node, metastasis) staging system is depicted in Table 75-2 and is acknowledged as the standard system for reporting outcomes in stomach cancer.58 Several comparison studies, including some from Japan,59,60 have shown better prediction of prognosis using the AJCC TNM system compared with other staging systems, including that of the Japanese Research Society for Gastric Cancer. Table 75-2 TNM Staging for Carcinoma of the Stomach Tis: carcinoma in situ; intraepithelial tumor without invasion of the lamina propria. T1: Tumor invades lamina propria, muscularis mucosa or submucosa. T1a: Tumor invades lamina propria or muscularis mucosa; T1b: Tumor invades submucosa T2: Tumor invades the muscularis propria T3: Tumor penetrates subserosal connective tissue without invasion of visceral peritoneum or adjacent structures. T4: Tumor invades serosa (visceral peritoneum) or adjacent structures. T4a: Tumor invades serosa (visceral peritoneum); T4b: tumor invades adjacent structures* N0: No regional lymph node metastasis. N1: Metastasis in 1–6 regional nodes. N2: Metastasis in 7–15 regional nodes. N3: Metastasis in more than 15 regional nodes. *Adjacent structures include the spleen, transverse colon, liver, diaphragm, pancreas, abdominal wall, adrenal gland, kidney, small intestine, and retroperitoneum. From Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th ed. New York: Springer Verlag; 2010. p. 117. Surgical excision has traditionally been the first treatment for gastric carcinoma. Because many patients have occult distant metastases at presentation and improved results have been demonstrated with preoperative chemotherapy61,62, neoadjuvant treatment is appropriate when preoperative staging is consistent with ≥T3 primary tumors and/or ≥N1 disease. The increasing prevalence of proximal gastric cancers, especially in the developed world, has resulted in more operations encompassing a thoracic component of resection to obtain an adequate proximal margin and remove intrathoracic lymph nodes at risk for metastasis. Surgical excision of the gastric and nodal components of disease remains the primary therapy for all potentially curable gastric carcinomas. Based on pathological findings, the Japanese Research Society for Gastric Cancer has defined four categories of surgical resection: (1) absolute curative (no peritoneal or hepatic metastases, no serosal involvement, and a level of lymph nodes removed beyond those involved); (2) relative curative (same as category 1 but nodal involvement to the level excised); (3) relative noncurative (complete gross tumor excision but curative criteria not met); and (4) absolute noncurative (residual cancer).50 Most curable tumors can be removed with adequate margins by subtotal gastrectomy; total gastrectomy is used when mandated by proximal cancer location or disease extent. Routine total gastrectomy does not improve survival by providing wider margins and eliminating multicentric disease but it may increase the rates of patient morbidity and mortality. A randomized study63 showed similar survival rates with subtotal and total gastrectomy. Surgical resection alone, including endoscopic mucosal resection in selected patients,64
Cancer of the Stomach and Gastroesophageal Junction
Epidemiology
Etiology and Biological Characteristics
Etiology
Biological Characteristics
Prognostic Factors
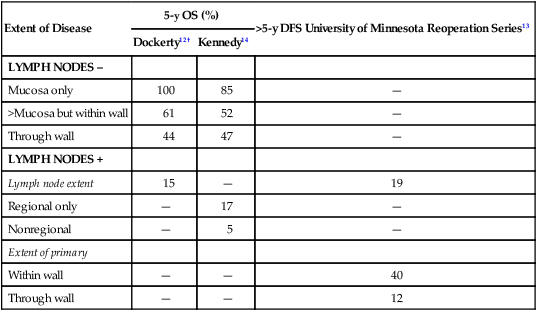
Extent of Disease
5-y OS (%)
>5-y DFS University of Minnesota Reoperation Series13
Dockerty12†
Kennedy14
LYMPH NODES –
Mucosa only
100
85
—
>Mucosa but within wall
61
52
—
Through wall
44
47
—
LYMPH NODES +
Lymph node extent
15
—
19
Regional only
—
17
—
Nonregional
—
5
—
Extent of primary
Within wall
—
—
40
Through wall
—
—
12
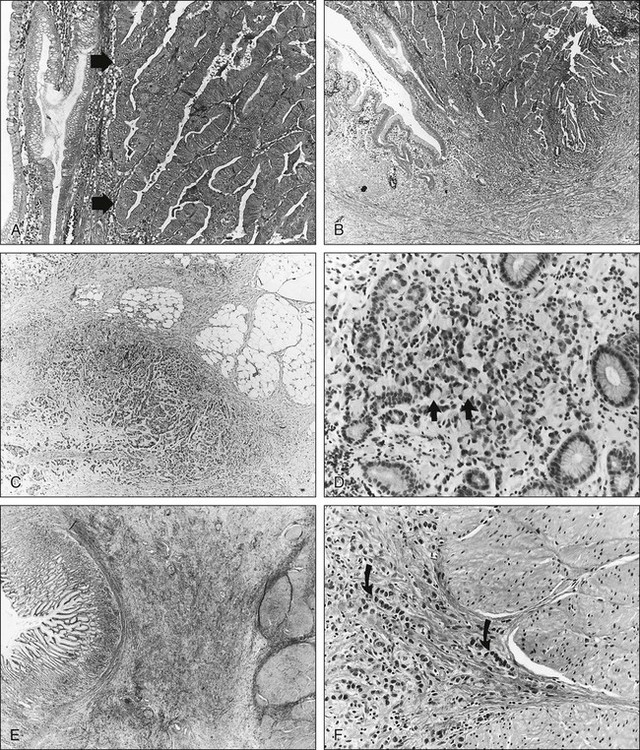
Molecular Biology
Prevention and Early Detection
Pathology and Pathways of Spread
Pathways of Tumor Spread
Direct Extension
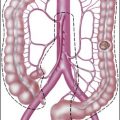
Lymphatics
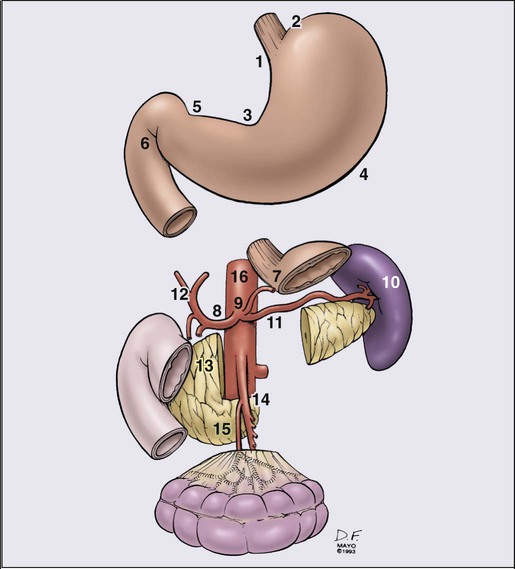
Clinical Manifestations, Patient Evaluation, Staging
Evaluation of the Patient
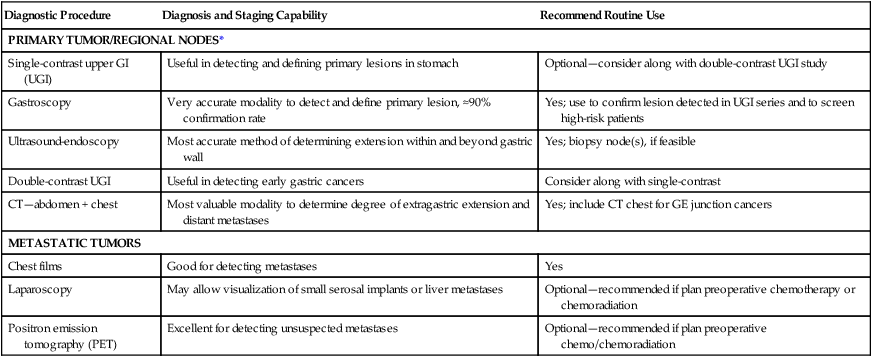
Diagnostic Procedure
Diagnosis and Staging Capability
Recommend Routine Use
PRIMARY TUMOR/REGIONAL NODES*
Single-contrast upper GI (UGI)
Useful in detecting and defining primary lesions in stomach
Optional—consider along with double-contrast UGI study
Gastroscopy
Very accurate modality to detect and define primary lesion, ≈90% confirmation rate
Yes; use to confirm lesion detected in UGI series and to screen high-risk patients
Ultrasound-endoscopy
Most accurate method of determining extension within and beyond gastric wall
Yes; biopsy node(s), if feasible
Double-contrast UGI
Useful in detecting early gastric cancers
Consider along with single-contrast
CT—abdomen + chest
Most valuable modality to determine degree of extragastric extension and distant metastases
Yes; include CT chest for GE junction cancers
METASTATIC TUMORS
Chest films
Good for detecting metastases
Yes
Laparoscopy
May allow visualization of small serosal implants or liver metastases
Optional—recommended if plan preoperative chemotherapy or chemoradiation
Positron emission tomography (PET)
Excellent for detecting unsuspected metastases
Optional—recommended if plan preoperative chemo/chemoradiation
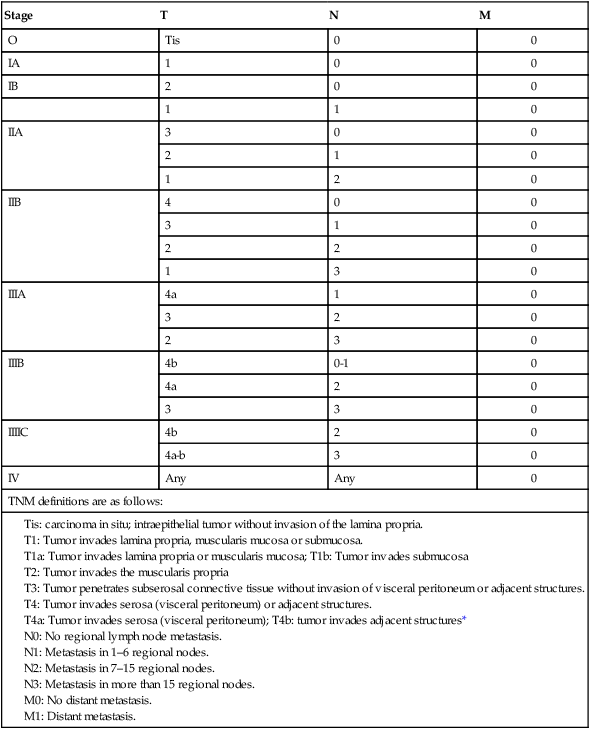
Staging
Stage
T
N
M
O
Tis
0
0
IA
1
0
0
IB
2
0
0
1
1
0
IIA
3
0
0
2
1
0
1
2
0
IIB
4
0
0
3
1
0
2
2
0
1
3
0
IIIA
4a
1
0
3
2
0
2
3
0
IIIB
4b
0-1
0
4a
2
0
3
3
0
IIIIC
4b
2
0
4a-b
3
0
IV
Any
Any
0
TNM definitions are as follows:
Primary Therapy
Surgical Method—Gastric Cancer
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Oncohema Key
Fastest Oncology & Hematology Insight Engine