37 Cancer of the Stomach
Epidemiology, Etiology, Genetics, and Cytogenetic Abnormalities
Epidemiology
Gastric and gastroesophageal junction (GEJ) adenocarcinoma constitutes a major health problem worldwide. Gastric cancer is the fourth most prevalent malignancy and the second leading cause of cancer death worldwide.1 In 2008, cancer of the stomach has an expected incidence in the United States of 21,500 cases (13,190 men and 8,310 women) and 10,880 deaths.2
Although there has been an overall reduction in gastric cancer incidence in both high and low-incidence countries, the incidence of adenocarcinoma of the gastroesophageal junction and gastric cardia has risen faster than any other malignancy in the last 25 years in the United States and other Western countries.3 The intestinal subtype is seen more commonly in older patients whereas the diffuse type affects younger patients and has a more aggressive clinical course. In a number of studies, the decrease in incidence has been attributed to the decline in the intestinal subtype. However, epidemiologic studies indicate that the diffuse subtype, especially signet ring cell type, is increasing.4
Etiology
Environmental Risk Factors
No single dietary agent has been determined to be the causative factor of gastric cancer. However, in case-controlled studies, gastric cancer appears to be positively correlated with the ingestion of pickled vegetables, salted fish, excessive dietary salt, and smoked meats.5–7 Fruits and vegetables may have a protective effect.8,9
Infectious Risk Factors
Helicobacter pylori is associated with inflammatory conditions in the stomach, in particular chronic atrophic gastritis. Although H. pylori by itself cannot cause gastric cancer, a growing body of evidence, including meta-analysis, suggests that it plays an important role in the development of adenocarcinoma of the stomach.10 Additionally, an association between Epstein Barr virus (EBV) and gastric cancer has been observed in 10% of cases, leading to speculation that this virus may also play a role in the development of gastric cancer, although further studies are needed for validation.11
Genetic Risk Factors
The genetic basis of gastric cancer and its precursors is being actively investigated. Emerging data suggest that the development of gastric cancer, similar to many other epithelial-derived carcinomas, is a multistep process that involves a successive activation or deletion of genes and their protein products that can occur through both genetic and epigenetic mechanisms. This is mediated by regulatory genes. Advances in molecular biology techniques have allowed characterization of the genetic changes thought to be responsible for this multistep process.12
One gene that has been shown to be critical in the development of gastric cancer is CDH1, that encodes E-cadherin, an epithelial cell adhesion molecule. Over half of gastric cancers have inactivated CDH1, through either genetic or epigenetic mechanisms. Germline CDH1 mutations have been identified in families with hereditary diffuse gastric cancer (HDGC), an example of a rare cancer susceptibility syndrome that has been very informative regarding cancer genetics and clinical approaches to a high risk for cancer. HDGC is an autosomal dominant inherited cancer syndrome clinically characterized by (1) two or more documented cases of diffuse gastric cancer (DGC) in first/second degree relatives, with at least one diagnosed before the age of 50, or (2) three or more cases of documented DGC in first/second degree relatives, independent of age of onset. The average age of onset of gastric cancer in affected families is 38, however individuals as young as 14 have died of the disease. HDGC is caused by the inheritance of a germline CDH1 mutation, typically a truncating mutation. Within the gastric mucosa a somatic event, usually CDH1 promoter methylation or an inactivating point mutation, provides the “second hit” leading to complete loss of E-cadherin function. Overall penetrance of diffuse gastric cancer in patients carrying a CDH1 mutation is estimated at 83% for women and 67% for men. Women with CDH1 mutations carry an additional 20-40% risk of lobular breast cancer.13
One challenge in managing a patient with a known CDH1 mutation is the inadequacy of current screening modalities. Diffuse gastric cancers found in HDGC patients are characterized by multiple infiltrates of malignant signet-ring cells which may underlie the normal mucosa. The wide distribution and small size of the malignant foci make them difficult to identify with random endoscopic biopsy. Because of high cancer penetrance, poor outcomes and inadequacy of clinical screening in HDGC, gene-directed prophylactic total gastrectomy is now offered to carriers of germline CDH1 mutations. In one published series of CDH1 mutation carriers who have undergone prophylactic gastrectomy, all specimens were found to contain multiple foci of diffuse, signet-ring cell cancer. DGC found in asymptomatic CDH1 carriers is typically early-stage and completely resected by prophylactic gastrectomy, so surgery can be considered curative.14
Other Risk Factors
Limited data suggest that radiation delivered for benign disease may be a risk factor for gastric cancer. In the earlier part of the 20th century, gastric irradiation was used to suppress gastric acid secretion. Orthovoltage radiation doses ranging from 1500 R to 2000 R in 10 days were used before the development of effective pharmacologic means to treat duodenal ulcers.15 In a small cohort of patients described by Peters and colleagues, patients who underwent partial gastrectomy and external-beam radiation therapy had an increased incidence of gastric cancer.16 Griem and associates reported on 1831 patients with peptic ulcer disease who were treated with radiation and compared them with a similar group of medically managed patients for an average of 22 years. Radiation therapy was linked to an increased relative risk for cancers of the stomach (RR = 2.77, 95% confidence interval), as was partial gastrectomy (RR = 2.60, 95% confidence interval). When surgery was combined with radiation therapy, the risk increased 10-fold.17 These results were only apparent after extended follow-up.18
A variety of other causal agents have been identified such as previous gastric surgery, adenomatous polyps, and chronic atrophic gastritis.12
Pathologic Conditions
The most common histologic condition (90%) is adenocarcinoma. Subtypes include papillary, tubular, mucinous, signet ring cell, adenosquamous, and squamous cell. Other histologic conditions include sarcoma, carcinoid, and small cell and undifferentiated carcinomas. Lymphomas (mucosa-associated lymphoid tissue [MALT]) and leiomyosarcomas can occur, albeit much more infrequently. MALT lymphomas are discussed in Chapter 54.
Jarvi and Lauren, based on epidemiologic studies, divided stomach cancers into two main groups: intestinal (expansile) or diffuse (infiltrative). In 1977, Ming proposed a modification of the Jarvi-Lauren classification.19 He emphasized the growth patterns rather than the tumor’s architectural subtypes. The classification divides tumors into two main patterns, expanding and infiltrative, which roughly correspond to the intestinal and diffuse types, respectively. The expanding pattern is more common in his series (67%) and describes tumors that tend to be fungating, whereas the infiltrating tumors tend to be diffuse.
Borrmann classified stomach cancer into four categories based on the gross morphology. These include type I: polypoid, type II: ulcerative, type III: ulcerating and infiltrating, and type IV: infiltrating. Type IV corresponds to the appearance of linitis plastica, in which infiltration by tumor causes rigidity of the gastric wall. Borrmann types I and II have a more favorable prognosis than Borrmann types III and IV, independent of lymph node involvement. Similarly, the Japanese Research Society for Gastric Cancer has a classification system based on gross appearance and divides lesions into protruded (I); superficial (II), with elevated (IIa), flat (IIb), and depressed (IIc) subtypes; and excavated (III) types.24
Diagnostic and Staging Studies
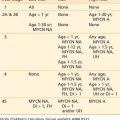
The workup for gastric cancer is described in Table 37-1. Numerous tumor markers have been described in association with gastric cancer, including CEA carcinoembryonic antigen) and Ca 19-9. However, the clinical utility of these tumor markers for screening, diagnostic staging, monitoring response to therapy, or as predictors of recurrence is controversial. Carcinoembryonic antigen (CEA) is positive in approximately one third of gastric carcinomas and reflects the extent of tumor burden. Postoperative elevated values of CEA are not helpful in predicting recurrence.
Table 37-1 Diagnosis and Staging for Gastric Cancer
Endoscopy
Flexible fiberoptic endoscopy with biopsy is more than 90% accurate in diagnosing gastric cancer.20 The positive yield per biopsy is higher in exophytic tumors than in nonexophytic tumors. In the advanced infiltrating lesion (Borrmann type IV), the diagnosis by biopsy is less accurate. The location of the tumor can affect the diagnostic accuracy. It is particularly difficult to obtain a tissue diagnosis from lesions in the gastric cardia and antrum located behind the incisure angularis. Numerous studies have examined the correlation between radiologic diagnosis, endoscopy, and biopsy. No method alone is usually 100% reliable. The proper combination of imaging and endoscopy results in the highest number of positive diagnoses. Such information helps the endoscopist direct the biopsy to suspicious areas in which there may not be mucosal abnormalities.
The application of endoscopic ultrasonography (EUS) allows the identification of the five gastric wall layers.21 In staging gastric tumors, EUS diagnoses the depth of penetration of the primary gastric carcinoma with approximately 90% accuracy.22 EUS can also be used to stage lymph node metastases. However, the EUS is is less accurate compared with the T stage. In one series, EUS had an accuracy of 83%, sensitivity of 54%, and a specificity of 97%. The positive predictive value was 88% and the negative predictive value was 82%.
Imaging
The use of positron emission tomography with fluorodeoxyglucose (FDG-PET) is currently considered an optional component in the workup of gastric cancer. Recent studies have shown some added benefit in preoperative staging, predicting response to preoperative chemotherapy, and for the evaluation of recurrent gastric cancer.23 The role of FDG-PET in the evaluation of metastatic gastric cancer is unclear. Combination PET-CT may provide advantages over PET alone. Further studies are needed to validate this imaging modality in the evaluation of gastric cancer.24
Staging System
In 1988 the American Joint Commission on Cancer (AJCC) and the Union Internationale Contre le Cancer proposed a joint TNM staging system. This was based on the fifth edition of the AJCC TNM staging system. The sixth edition of the AJCC TNM staging system is seen in Table 37-2.25 The major change from the fifth edition is that T2 lesions have been divided into T2a (tumor invades the muscularis propria) and T2b (tumor invades the subserosa). There are also additional descriptors that, although they do not affect the stage grouping, do indicate cases needing separate analysis.
As with most other gastrointestinal malignancies, T stage is based on the extension through the layers of the stomach. Lymph node staging is based on the number of positive regional lymph nodes. Whether a lymph node is staged as nodal (N) or metastatic (M) disease depends on the relationship of the nodes to the primary tumor. In general, regional nodes include those located in the greater and lesser curvatures, as well as those in the left gastric, common hepatic, splenic, and celiac arteries. Involvement of other intra-abdominal nodes, including hepatoduodenal, retropancreatic, mesenteric, and paraaortic, are staged as metastatic disease. A list of specific regional and distant nodal disease is seen in Table 37-2. Patients with Epstein-Barr virus–involved gastric cancers have a lower incidence of positive nodes.26
Standard Therapeutic Approaches
Surgery
The primary treatment for local and regional gastric cancer is surgery. An analysis from the U.S. National Cancer Database of 50,169 patients treated from 1985 through 1996 who underwent a gastrectomy as a component of their therapy revealed a decrease in overall 5-year survival rate with increasing stage: IA, 78%; IB, 58%; II, 34%; IIIA, 20%; IIIB, 8%; and IV, 7%.27 In most cases, the determination of resectability for cure or palliation can only be assessed at the time of surgical exploration. The specific surgical procedure is based on the location and extent of the primary tumor and the respective lymph node drainage areas. Laparoscopy can detect disease in the peritoneal cavity and is helpful prior to a laparotomy.28 The most common site of failure is the abdomen.29
Subtotal versus Total Gastrectomy
The first randomized prospective trial comparing subtotal with total gastrectomy for distal gastric cancers was reported by Gouzi and colleagues.30 Both groups had similar morbidity (33%), mortality (1.3% versus 3.2% respectively), and 5-year survival (48%). Therefore, although elective total gastrectomy can be performed safely, subtotal and total gastrectomy offer equal long-term results in patients with carcinomas of the distal stomach. Most surgeons in the United States recommend a subtotal gastrectomy, providing that negative margins and an adequate lymphadenectomy can be achieved. Proper lymph node staging requires examination of 10 to 15 nodes.31 Negative margins usually require transection distally in the duodenum and at least 6 cm proximal to the palpable tumor. Margins must be confirmed with histopathology.
Conventional versus Extended Lymph Node Dissection
Lymphatic flow in the stomach is complex. Regardless of the location of the primary tumor, numerous nodal groups can be involved from either lymphatic spread occurring in a sequential manner or with metastases to distant lymph nodes without evidence of involvement of perigastric nodes (skipping). In rules established by the Japanese Research Society for Gastric Cancer, the lymph node groups are numbered 1 through 16, and are grouped into four lymph node levels or groups, N1 through N4. The determination of which nodal stations are placed in which nodal groups depends on the location of the primary tumor. By convention, N1 and N2 are considered regional nodes, whereas metastasis to N3 and N4 node groups is considered distant metastasis. Most Japanese surgeons recommend an extended dissection. The rationale is that improved local regional control results in improved patient survival. However, the data that support this hypothesis are controversial. In Japan, there is widespread endoscopic screening, and public health campaigns resulted in the diagnosis of a larger proportion of early stage tumors. The cure rates reported by Japanese investigators may be more related to the reduced stage of disease than the more radical surgical approach.32 Such “stage migration” may be responsible for the improved survival in each stage of the disease.
Three randomized trials have addressed the question of the extent of surgery.33–35 The three trials confirm that not only was there not an improvement in survival with more radical surgery (D2 versus D1 versus D0), but there was a corresponding increase in the incidence of complications. For example, in the Dutch trial, compared with D1 resection, patients who underwent a D2 resection had a significantly higher incidence of complications (43% versus 25%, p < 0.001), mortality (10% versus 4%, p = 0.004), and no difference in 11-year survival (35% versus 30%, p = 0.53).33 Almost all agree that a D0 resection is not adequate, and, in general, the recommended operation is at least a D1 for lesser curvature lesions and a full D2 for greater curvature lesions. The effect of a D2 resection on the results of the intergroup trial (INT) 0116 of postoperative adjuvant combined modality therapy36 is discussed in the “Outcomes” section of this chapter.
Sasako and colleagues randomized 523 patients with stages T2b through T4 gastric cancer to D2 resection with or without a prophylactic para-aortic lymph node dissection.37 There was no significant difference in 5-year survival (69% versus 70%, respectively).
Chemotherapy
The use of chemotherapy in the treatment of gastric cancer has been evaluated in a number of settings: neoadjuvant, adjuvant after complete resection, and for locally advanced or metastatic disease. Unfortunately, there are no accepted international standards for the optimal timing of chemotherapy or the combination of chemotherapeutic agents selected, leaving the decision to the provider. Two recent randomized trials reveal a significant improvement in survival with perioperative chemotherapy without radiation. Cunningham and colleagues reported the results of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial in which 503 patients with clinical stage II and III gastric cancer (11% GE junction) were randomized to three cycles of perioperative etoposide/cisplatin/5-Fluorocil (ECF) followed by surgery and three cycles of postoperative ECF, versus surgery alone.38 Approximately 40% of patients underwent a D2 resection. There was a significant improvement in 5-year survival in those who received chemotherapy versus observation (36% versus 23%, p = 0.009) The efficacy of the oral fluoropyrimidine S-1 has been reported in a randomized trial by Sakuramoto and colleagues. All patients underwent a resection (including D2 lymph node resection), and if they had stage II or III disease (89% were lymph node–positive) were randomized to surgery alone versus adjuvant S-1.39 Compared with surgery alone, those who received S-1 had a significant improvement in 3-year survival (80% versus 70%, HR 0.68, 95% CI 0.52–0.87, p = 0.003). Although local failure was 3% and nodal failure 9%, there was no significant improvement in distant control in patients receiving S-1.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree