66 Cancer of the Skin
Epidemiology, Etiology, and Pathogenesis of Basal and Squamous Cell Carcinomas
Nonmelanoma skin cancer represents the most common malignancy in humans. In the United States, the incidence of nonmelanoma skin cancer approaches that of all noncutaneous cancers combined. According to the American Cancer Society, more than 1 million new basal and squamous cell carcinomas of the skin occurred in the United States in 2008,1 with one in five Americans developing skin cancer during their lifetime.2 As cases are often not reported to cancer registries, the actual incidence of these cancers likely far exceeds these estimates. Despite increased knowledge and public education regarding the causes of skin cancer and modes of prevention, the incidence of nonmelanoma cutaneous cancer continues to rise. The cause of this phenomenon is largely speculative but is believed to be due to changes in both sun exposure habits among patients and improved detection. Some researchers have also hypothesized that stratospheric ozone depletion may be a contributing factor.3
Basal cell carcinomas and squamous cell carcinomas account for approximately 80% and 20% of nonmelanoma skin cancer, respectively.4 The term nonmelanoma skin cancer is often used to refer to these two histologies; however, other types of nonmelanoma skin cancer, such as adnexal carcinomas (involving the sweat and sebaceous glands) and sarcomas, are less common and differ considerably in their cell type, behavior, and epidemiologic features. Although basal cell carcinoma rarely metastasizes, the morbidity associated with this type of skin cancer can be significant. Cutaneous squamous cell carcinoma carries an increased risk of metastasis and an overall worse prognosis. An estimated 1000 to 2000 deaths result each year from nonmelanoma skin cancer in the United States, with population-based mortality rates of approximately 0.05% and 0.7% for basal cell carcinoma and squamous cell carcinoma, respectively.5
Because damage from ultraviolet (UV) light is the most common (and preventable) predisposing factor, there is a relatively low incidence of skin cancer in dark-skinned people, who are protected by the large amount of melanin in their skin. On the other hand, there is a relatively high incidence in whites of Irish, Scottish, or English descent who have red or blond hair and blue or green eyes and who burn rather than tan when in the sun. Damage to the skin from UV light appears to be cumulative with time. Consequently, skin cancer usually develops after the age of 40 years.6–10 The incidence of nonmelanoma skin cancer correlates with skin phenotype, the amount of average annual UV radiation, and geographic latitude, with the highest incidences reported in countries such as Australia and South Africa.8,11–19 Trauma and chronic irritation are additional risk factors. Skin cancers have been reported in scars resulting from vaccinations,20 tattoos,21 burns (Marjolin ulcer),22–24 and chickenpox25; in chronic nonhealing ulcers26; and at sites of prior ionizing radiation therapy and chronic radiation dermatitis.27 Other predisposing clinical settings include longstanding lesions of discoid lupus erythematosus,28–30 erosive or hypertrophic lichen planus,31 lichen sclerosus32; porokeratosis33,34; and nevus sebaceous.35 Occupational exposures to arsenic-containing pesticides, mineral oil, coal tar, soot, and polychlorinated biphenyls are associated with an increased risk for the development of nonmelanoma skin cancer.36 Another recent study suggested that the use of tanning beds significantly contributes to the incidence of nonmelanoma skin cancer.37
Oncoviruses have also been implicated.38 Genetic disorders such as xeroderma pigmentosum,39 oculocutaneous albinism,40 epidermodysplasia verruciformis,41 dystrophic epidermolysis bullosa,34 nevoid basal cell carcinoma syndrome (NBCCS; Gorlin syndrome),42 Bazex syndrome,43 and Rombo syndrome44 are associated with an increased incidence of skin cancer.
Iatrogenic immunosuppressive therapy,45 especially in the organ transplant population,46,47 is an increasingly prevalent risk factor for developing skin cancer, and in particular squamous cell carcinoma. Organ transplant patients appear to have a 65-fold increased risk of squamous cell carcinoma and a 10-fold increased risk of basal cell carcinoma.48,49 Similarly, patients with acquired immunodeficiency syndrome (AIDS),50 leukemia, lymphoma, or multiple myeloma have an increased risk of developing skin cancer.51 Persons with AIDS have a three- to fivefold increased risk of nonmelanoma skin cancer; in contrast to organ transplant recipients, the ratio of squamous cell carcinoma to basal cell carcinoma is approximately 1 : 7.52 Although basal cell carcinomas can be multiple in the human immunodeficiency virus (HIV) population, they are most often of the superficial subtype. Squamous cell carcinoma in HIV may be associated with younger age and in certain clinical circumstances, unusual human papillomavirus subtypes (anogenital, cervical, oral, and nail unit squamous cell carcinomas, and HIV-associated epidermodysplasia verruciformis). There is also a higher risk for aggressive behavior, seemingly unrelated to CD4 count. In general, skin cancers in immunosuppressed populations are more likely to be advanced; this subset of patients deserves close clinical monitoring.
Germline mutations in the human homolog of the Drosophila patched gene (PTCH) were first implicated in the pathogenesis of NBCCS (Gorlin syndrome) in 1996; in NBCCS, patients develop multiple and recurrent basal cell carcinomas beginning at a young age.53,54 Disruptions in the Hedgehog signaling pathway, which under basal conditions appears to be essential for normal embryonic development and tumor suppression, have since been found in sporadic basal cell carcinomas as well.55–57 Hedgehog pathway mutations are the most frequent genetic alterations in basal cell carcinomas and are likely sufficient for tumorigenesis. Mutations in the p53 tumor suppressor gene are commonly found in both basal and squamous cell carcinomas.58
Anatomy of the Skin
The three layers that make up the skin are the epidermis, dermis, and subcutaneous tissue.36 The epidermis is a stratified squamous epithelium that is approximately 0.05 mm thick,59 and composed of the stratum basale, stratum spinulosum, stratum granulosum, and stratum corneum. In normal skin, transit time of a basal keratinocyte through the stratum corneum is approximately 4 weeks.60 The basement membrane zone separates the epidermis from the underlying dermis. The dermis, which is 1 to 2 mm thick, contains lymphatics, nerves, blood vessels, sweat, and sebaceous glands in a connective tissue stroma, and serves to provide both structural and nutritional support for the epidermis. The superficial portion of the dermis in continuity with the epidermis is called the papillary layer because of its projecting dermal papillae. The deeper portion of the dermis, which contains bundles of collagenous and elastic fibers, is called the reticular layer. Subcutaneous tissue lies deep to the dermis and supports the lymphatics, blood vessels, and nerves in loose connective tissue containing variable amounts of fat.
Pathologic Conditions and Clinical Presentation
Basal Cell Carcinoma Subtypes
There are several subtypes of basal cell carcinoma that differ in clinical presentation, histologic appearance, and behavior.37,61 Of the most common subtypes, nodular and superficial basal cell carcinomas typically demonstrate low-risk clinical behavior, whereas infiltrative, morpheaform, and micronodular basal cell carcinomas are more likely to recur, and be locally aggressive and destructive.
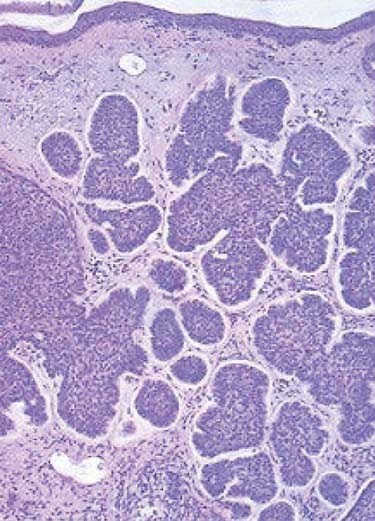
The nodular subtype, which accounts for approximately one half of all basal cell carcinomas, initially appears as a pink, translucent papule with prominent telangiectasias. As the carcinoma grows, it may develop central ulceration and crusting (“rodent ulcer”). Histologically, all basal cell carcinomas share common features including proliferations of basaloid keratinocytes in various configurations, often with apparent epidermal attachment, an associated fibromyxoid stroma, and tumoral-stromal clefting. The malignant cells in nodular basal cell carcinoma form dermal nests.62 At the periphery of the nests, the basaloid cells elongate in a parallel array, forming a palisading pattern. The malignant cells exhibit little pleomorphism, and mitoses are infrequent (Fig. 66-1). The clinical differential diagnosis of nodular basal cell carcinoma includes rosacea, acne, folliculitis, angiofibroma, benign adnexal tumors, and granulomatous disorders such as sarcoidosis and granuloma annulare. The superficial subtype, which accounts for one third of basal cell carcinomas, typically appears as a red, scaly macule or thin plaque located on the trunk or extremities. Histologically, the basaloid proliferations are smaller and more superficially located. The differential diagnosis includes actinic keratosis, squamous cell carcinoma in situ (Bowen disease), extramammary Paget disease, or a benign inflammatory lesion of psoriasis, tinea corporis, or nummular eczema. Infiltrative and morpheaform basal cell carcinomas present as light-colored atrophic macules that later become smooth, shiny, red to white, indurated plaques resembling scars, often with overlying telangiectasia. The margins of infiltrative and morpheaform basal cell carcinomas are difficult to assess clinically. Although the terms infiltrative and morpheaform are often used as synonyms, some authors prefer the use of infiltrative to describe tumors with jagged nests, and morpheaform for tumors in which thin strands of basaloid cells predominant within a fibromyxoid stroma. The differential diagnosis includes scleroderma, metastatic carcinoma, scar, and granulomatous disorders. Infiltrative and morpheaform subtypes account for 10% of basal cell carcinomas. Micronodular basal cell carcinomas may present nonspecifically as macules, papules, or thin plaques. On histopathologic examination, basaloid tumor nests are smaller than those of nodular basal cell carcinoma, but as compared with superficial basal cell carcinoma, may invade deeply. The pigmented subtype, which accounts for 1% of basal cell carcinomas and is considered by some authors a variant of nodular basal cell carcinoma, ranges in color from blue to black and can be difficult to distinguish from melanoma. This subtype occurs more often in darkly-pigmented individuals. Fibroepithelioma of Pinkus, which accounts for less than 1% of basal cell carcinomas, presents as a flesh-colored, often pedunculated papule or plaque on the trunk or thighs. Histopathologic examination reveals thin anastomosing strands of tumor cells that extend from the epidermis, and are enmeshed in a fibrous stroma. Basosquamous carcinomas (metatypical carcinomas or keratotic basal cell carcinomas) occur almost exclusively on the face. These are uncommon neoplasms that demonstrate areas of squamous differentiation and whose clinical behavior sometimes resembles that of squamous cell carcinomas.
Squamous Cell Carcinoma Subtypes
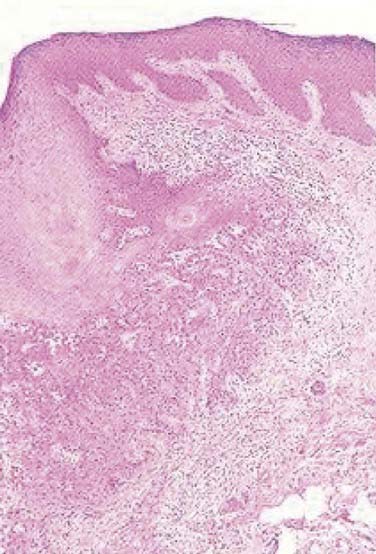
On histopathologic evaluation, Bowen disease demonstrates full-thickness epidermal atypia, with more pronounced nuclear polymorphism and apoptosis than is seen in actinic keratosis. Other features include confluent parakeratosis, and, not infrequently, the adnexal extension of neoplastic cells. Invasive squamous cell carcinoma typically arises on a background of epidermal change consistent with actinic keratosis, and, less often, squamous cell carcinoma in situ. Invasive tumor lobules push downward from the overlying epidermis and detached tumor islands are noted within the dermis (Fig. 66-2). Both cytoplasmic and cystic keratinization may be observed. The degree of keratinocyte differentiation within these tumors is variable and an important prognostic factor. Other histopathologic risk factors include tumor thickness and depth of invasion, perineural involvement, lymphovascular invasion, and subtype. Pathologic subtypes associated with clinically aggressive behavior include adenoid (pseudoglandular), acantholytic, adenosquamous, and desmoplastic squamous cell carcinoma.
A few additional subtypes are clinically distinctive and noteworthy. Verrucous carcinoma is an indolent, well-differentiated squamous cell carcinoma that grows slowly as an exophytic, cauliflower-like lesion and may be associated with human papilloma virus infection. This variant of squamous cell carcinoma may arise in the anogenital region (Buschke-Lowenstein tumor), oral cavity (oral florid papillomatosis), or on the plantar surface of the foot (epithelioma cuniculatum). Spindle cell carcinoma, a rare subtype of squamous cell carcinoma, usually develops in sun-exposed areas in lightly-pigmented individuals older than 40 years of age.63 The prognosis primarily depends on the depth of invasion. Verrucous and spindle cell carcinomas are managed similar to more conventional squamous cell carcinomas.
Although keratoacanthomas were historically considered benign secondary to a natural history of spontaneous regression in some cases, they are currently viewed by most authors as a variant of squamous cell carcinoma. A keratoacanthoma presents as a rapidly enlarging papule that becomes a crateriform nodule with a central keratinous plug over a period of weeks to months. Some lesions may regress slowly over months to form an atrophic scar. Although keratoacanthomas are most often solitary, numerous clinical variants have been described, including multiple, grouped, giant, subungual, or intraoral lesions. Unusual presentations include keratoacanthoma centrifugum marginatum (a lesion that expands extensively to the periphery with central involution and atrophy), multiple spontaneously regressing (Ferguson-Smith), multiple nonregressing, and generalized eruptive (Grzybowski) keratoacanthomas. Multiple keratoacanthomas in association with sebaceous neoplasms, colon carcinoma, and other visceral malignancies characterize Muir-Torre syndrome. In most clinical situations, keratoacanthomas are best treated similar to conventional squamous cell carcinoma. Although intralesional FU or intralesional methotrexate may be used for multiple lesions, surgical excision is the most commonly performed procedure for a solitary keratoacanthoma. However, at sites such as the pinna of the ear, eyelid, nasal tip, nasal ala, or commissure of the lips, radiation therapy may provide better cosmetic and functional results. Shimm and colleagues described the treatment of 13 keratoacanthoma patients with radiation therapy.64 With a median follow-up of 41 months, the local control rate was 100% and the cosmetic results were excellent. For keratoacanthomas of 2 cm or less, Shimm and colleagues recommend a total dose of 25 Gy in five fractions on consecutive days, using superficial or orthovoltage x-rays. “Giant” (>2 cm) keratoacanthomas can be successfully managed with a total dose of 50 Gy in 15 fractions over 3 weeks using electrons.65,66
Routes of Spread
During weeks 4 to 8 of embryologic development, embryologic fusion planes are postulated to form at the junctions of various anatomic regions. Although embryologic fusion planes were traditionally thought to dictate the depth of invasion, horizontal growth, and area of recurrence of epithelial carcinomas,67–69 controversy has arisen as to whether these planes persist in adults and influence the spread of skin cancers.70,71
The perineural space represents a cleavage plane between a nerve and its surrounding sheath, and a pathway of apparently decreased resistance for the spread of skin cancer.72 Although small superficial cutaneous nerves are not uncommonly involved on histopathology in both squamous cell and basal cell carcinoma, large named nerve trunks are much less frequently affected.73 As the literature has often failed to differentiate between small and large nerve, microscopic and macroscopic (or clinical) perineural invasion, accurate estimates of incidence in each case are difficult to ascertain. Perineural inflammation in association with so-called skip areas, where no cancer cells are identified on the pathologic specimen,74 may be a clue to the presence of perineural tumoral invasion on more proximal sections of nerve.74,75 Notably, 60% to 70% of patients with pathologic evidence of perineural invasion by a basal or squamous cell carcinoma are asymptomatic.76–79 Once compression of a nerve or, less commonly, intraneural invasion72,77,80 develops, a patient is more likely to become symptomatic with anesthesia, paresthesia (formication, burning, stinging or shooting pain), facial muscle weakness, ptosis, diplopia, blindness, or stroke.76,77,81,82
Mohs noted perineural invasion in 0.9% of 2488 basal cell carcinomas in 1978.83 Basal cell carcinomas with evidence of perineural involvement are most often located in areas innervated by peripheral branches of the trigeminal and facial nerves.75 Basal cell carcinomas with perineural invasion are less common than their squamous cell carcinoma counterparts and are typically advanced, recurrent, or rarely metastatic when perineural invasion is present.84,85 Estimates of the incidence of perineural invasion in squamous cell carcinoma have ranged from 2.4% to 14%.74,78,83 Although microscopic perineural invasion can be an incidental finding in squamous cell carcinoma, it may also indicate a higher risk of local recurrence and even regional metastasis.86,87 It is clear that squamous cell carcinomas with macroscopic or clinical perineural involvement are associated with a higher incidence of regional and distant metastases.86–89 When squamous cell carcinomas involve the cranial nerves (most often the trigeminal and facial nerves), there may be significant morbidity and mortality, especially in cases with extension to the skull base and subsequent intracranial spread.
Other potential routes of spread for cutaneous carcinomas include perichondrial, periosteal, tarsal, and fascial planes, as well as along blood vessels and lymphatics.67
Staging
Some authors have postulated that basal cell carcinoma rarely metastasizes because it uniquely depends on stroma produced by dermal fibroblasts.90 Accordingly, the incidence of metastatic basal cell carcinoma is thought to be quite low, between 1 : 1000 to 1 : 35,000, and staging is infrequently required.85 The rare basal cell carcinoma that does metastasize is typically a large, ulcerative tumor for which repeated treatments over the years have failed. The most common metastatic site for basal cell carcinoma is the regional lymph nodes, although the lungs, liver, or bones may also be involved.91
The incidence of regional lymph node metastasis in patients with squamous cell carcinoma of the skin varies widely in the literature. In general, small (<2 cm), thin (<4 mm), and primary squamous cell carcinomas are felt to be low-risk (<5%) for the development of nodal metastasis.92–94 Although high-risk cutaneous squamous cell carcinomas can be associated with significant morbidity and mortality and must be identified early so that appropriate management can be recommended, universal and validated staging criteria are lacking. The current American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system (Table 66-1) groups all nonmelanoma skin cancers together (excluding carcinomas of the eyelid, vulva, and penis), and does not incorporate many important prognostic factors for squamous cell carcinoma.95,96 High-risk features that do appear to be predictive of metastatic disease include size larger than 2 cm, site (lip, areas with parotid drainage, scalp), incomplete excision and recurrence, thickness and depth of invasion (>4-5 mm depth, Clark level >III), histologic grade, histologic variant, perineural invasion, lymphovascular invasion, and immunosuppression.78,89,92,96–110 Squamous cell carcinomas that arise in burn scars or osteomyelitic foci have also been shown to metastasize to lymph nodes at an increased rate, in 10% to 30% of cases.94 In light of the growing literature on high-risk cutaneous squamous cell carcinoma, revision of the AJCC staging system is underway. When a squamous cell carcinoma metastasizes to regional lymph nodes (usually parotid and cervical), surgery and postoperative radiation therapy provide better locoregional control than radiation therapy alone.111,112 The 5-year survival rate for patients with lymph node metastases is 25%.113 Like basal cell carcinomas, typical distant metastatic sites for squamous cell carcinomas are the lungs, liver, and bones.
Table 66-1 Classification of Carcinoma of the Skin (Excluding Eyelid, Vulva, and Penis) Rights were not granted to include this table in electronic media. Please refer to the printed book.
Diagnostic Studies
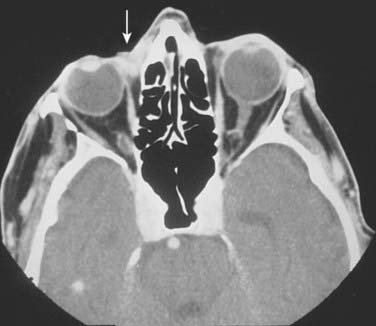
In the case of carcinomas involving the medial or lateral canthi of the eyes, one should consider obtaining either a computed tomography (CT) or magnetic resonance imaging (MRI) scan, to assess the depth of invasion, because apparently superficial cancers sometimes extend along the wall of the orbit (Fig. 66-3). If perineural invasion is present or suspected, MRI is the imaging study of choice to determine if retrograde spread of the carcinoma has occurred.114 However, in some cases, abnormal radiographic findings, for example, nerve or foramen enlargement, may not develop until late in the course of the disease.72,74,75 If there is suspicion that a locally advanced carcinoma has invaded underlying bone or metastasized to the regional lymph nodes, or distant sites, a CT, MRI, or positron emission tomography (PET) scan should be obtained. Notably, PET scans may offer superior sensitivity and specificity for the detection of metastases, when compared with more conventional imaging modalities such as CT or MRI. Adams and colleagues reviewed the detection of cervical lymph node metastases (117 metastases confirmed by histopathologic examination in 1284 lymph nodes) from head and neck carcinoma, and found that fluorine-18 fluorodeoxyglucose PET correctly identified nodal metastases with a greater sensitivity (90%) and specificity (94%), than either CT (sensitivity 82%, specificity 85%) or MRI (sensitivity 80%, specificity 79%).115
Treatment Options
Because most skin cancers can be treated with several different therapeutic approaches, it is important to select the most appropriate treatment. A number of criteria are useful in making this decision. Stratification of tumors into low-risk or high-risk categories should be performed. Consideration should be given as to whether single or multiple cancers are present. The age and general health of the patient, cure and complication rates, cosmetic and functional results, convenience, and cost of the different therapeutic approaches should also be discussed. For high-risk tumors, multispecialty consultation as well as preoperative imaging studies should be considered to facilitate the development of a rational therapeutic plan. The role of sentinel lymph node biopsy remains poorly defined.92 Metastatic carcinomas may need a combination of treatment modalities for local control and therapy of regional and distant metastases.
There are several forms of treatment for basal and squamous cell carcinomas, including cryosurgery, curettage and electrodesiccation, chemotherapy, surgical excision, Mohs micrographic surgery, and radiation therapy. Cryosurgery or cryotherapy involves fast freezing and slow thawing via the application of liquid nitrogen to the skin.116 Although cryosurgery is convenient and relatively inexpensive, it provides no therapeutic or cosmetic advantage over other more commonly used treatment modalities and thus its use for skin cancers should be considered largely historical. The technique involves freezing of the carcinoma to a goal tissue temperature of −70° C along with a 3- to 5-mm margin of normal tissue; the ice block is allowed to thaw, and the process is repeated one or two times to cause selective tumor necrosis. Adverse reactions include significant pain, crusting, edema, bulla formation, and skin sloughing. As melanocytes are particularly sensitive to cryosurgery, hypopigmentation is an expected complication. Contraindications include high-risk or recurrent tumors and patients with known cold urticaria, cryofibrinogenemia, cryoglobulinemia, or Raynaud disease. Relative contraindications include patients with darkly pigmented skin, tumors overlying superficial nerves or in anatomic regions subject to scar and retraction.
Curettage and electrodesiccation is commonly used for previously untreated superficial skin cancers. With a curette, the comparatively soft and friable cancer-containing tissues are scraped away, leaving behind firmer uninvolved tissue. After each curettage, the wound is electrodesiccated to encompass a 1-mm margin beyond the curetted tissue. This process is typically repeated for a total of three cycles. Patients must be cautioned that prolonged postoperative wound care will be required as the wound granulates and reepithelializes. Scarring is generally cosmetically acceptable, but may lead to a hypertrophic or hypopigmented scar at the site of treatment. Like cryosurgery, curettage and electrodesiccation is an effective method of managing primary superficial or nodular basal cell carcinomas; squamous cell carcinomas in situ; and small, well-differentiated squamous cell carcinomas with clinically distinct margins. As no pathologic specimen is generated to indicate complete treatment, this approach is not recommended for high-risk or recurrent tumors, carcinomas involving regions prone to scar with retraction, or carcinomas involving scar tissue, cartilage, or bone.117,118
Photodynamic therapy (PDT) involves the use of topical, intralesional, or systemic photosensitizers in combination with a photoactivating light source to create cytotoxic oxygen radicals and achieve tumoral tissue destruction. Topical PDT is typically limited to the treatment of multiple actinic keratoses or small superficial skin cancers.119–121 Curettage is often performed to debulk the tumor; this is followed by application of either 5(δ)-aminolevulinic acid HCl (ALA) and methyl-esterified ALA, and exposure to a variety of light sources. Side effects from topical PDT include mild pain, local site reactions, and sensitivity to ambient light for 2 to 7 days. Caution should be employed in patients with a history of porphyria or other photosensitivity disorders, and with prior or current herpes simplex virus infection.
Topical 5-FU chemotherapy is most often used to treat multiple actinic keratoses, superficial basal cell carcinoma, and squamous cell carcinoma in situ.119,122,123 Imiquimod cream, a Toll-like receptor 7 agonist, induces interferon-alpha and other T helper-1 cytokines, and may be used for similar indications.124–127 Although some authors have used topical imiquimod for the treatment of nodular basal cell carcinoma, cure rates are inferior when compared with superficial basal cell carcinoma.124,125 Both topical 5-FU and imiquimod are generally well-tolerated, with side effects limited to local skin reactions. Intralesional FU or methotrexate have been used for the treatment of keratoacanthomas.
Thus far there have been few reports regarding the use of systemic chemotherapy for basal and squamous cell carcinomas. Overall, the results achieved with other therapeutic approaches have been excellent, so systemic chemotherapy has been unnecessary in most patients. However, advanced skin cancers do respond to 5-FU, cisplatin, bleomycin, doxorubicin, isotretinoin, interferon-alpha, or a combination of agents. For patients treated with chemotherapy alone, response rates have characteristically ranged from 70% to 80%, with a complete response rate of 30%.128–130 These results are not surprising considering the high response rates that have been achieved when squamous cell carcinomas of the head and neck have been treated with two to three cycles of chemotherapy before radiation therapy.131 Approximately half the responses have lasted longer than 1 year.128 In the case of locally advanced or metastatic carcinoma of the skin, chemotherapy concurrent with radiation therapy may be considered.132 Further investigations are needed to evaluate the potential of capecitabine, an oral pro-drug of 5-FU with a more favorable safety profile than its parent drug,133 and the epidermal growth factor receptor inhibitors,134 which have recently emerged as novel treatment strategies for aggressive squamous cell carcinoma of the skin.
Advances in reconstructive techniques have helped make surgical excision a commonly used therapeutic approach. Standard surgical excision with predetermined margins allows pathologic assessment of the extent of a carcinoma via the bread-loaf technique of tissue sectioning, but assesses only 0.01% of the total margin and may be difficult in older patients with either a large solitary carcinoma or multiple small carcinomas in the same region.135 With Mohs micrographic surgery, horizontal layers of tissue are serially excised with a beveled edge, and systematically mapped.136 Frozen tissue sections are prepared, and close to 100% of the peripheral and deep margins are examined. If carcinoma is detected at the margin, another horizontal layer of tissue is excised from the area and examined microscopically. The process is repeated until no cancer is detected at the margin. With Mohs micrographic surgery, both superior histopathologic confirmation of tumor removal and tissue conservation are accomplished. Frederic Mohs, who first described this method in 1941,137 initially proposed that zinc chloride paste be applied to the skin as a fixative 24 hours prior to excision and sectioning of tissue (fixed-tissue technique). Later, the fixative was omitted (fresh-tissue technique) to do away with the pain associated with tissue fixation, allow multiple stages of surgery to be performed in one day, and leave a defect that could be repaired immediately if desired.138 Mohs micrographic surgery leaves a tissue defect that may require surgical repair; however, it is a highly effective approach for high-risk, incompletely excised, and recurrent skin cancers, as well as tumors located in areas where tissue conservation is of functional and cosmetic importance. Contraindications to Mohs surgery include cancers with discontiguous growth and difficult to diagnose features on frozen-section pathologic examination. Rowe and colleagues have reported 5-year cure rates of 99% and 97% with Mohs micrographic surgery for basal and squamous cell carcinomas respectively.98,139 Squamous cell carcinomas with perineural invasion should be treated with Mohs micrographic surgery and postoperative radiation therapy; for basal cell carcinomas with perineural invasion, post-Mohs micrographic surgery radiation therapy may be considered.74,75
Like Mohs micrographic surgery, radiation therapy constitutes an effective form of treatment for recurrent carcinomas as well as large carcinomas involving the forehead or scalp. Radiation therapy is also generally recommended for carcinomas located in the central face and is particularly well suited to the treatment of cancers (≥0.5 cm) involving the ears, eyelids, tip or ala of the nose, or commissure of the lips because these sites can be difficult to reconstruct surgically. Advantages of radiation therapy are that the treatment is noninvasive, relatively painless, and, for the sites outlined earlier, less expensive than Mohs micrographic surgery followed by reconstructive surgery. Excellent rates of disease control and cosmetic outcomes can be attained with careful attention to patient selection and radiation technique.140–143 Although morpheaform basal cell carcinomas normally are not managed with radiation therapy because the margins are frequently difficult to assess clinically, they are radioresponsive. When wide margins have been provided (see the section on field size), local control has been achieved with radiation therapy in patients who declined Mohs micrographic surgery.144–145
Indications for Postoperative Radiation Therapy
Studies with a minimum follow-up period of 5 years indicate that approximately one third of basal cell carcinomas that extend to the margin of resection ultimately recur.146–149 Consequently, the management of a positive margin is somewhat controversial. Because it may be difficult to detect early recurrences in cases in which a basal cell carcinoma extends to the deep margin and the area is fibrotic from prior treatment, where grafts have been used to close a surgical defect, or where close follow-up will not be possible, immediate retreatment with surgical excision, Mohs micrographic surgery, or radiation therapy should be considered.150–151 In cases involving a graft, radiation therapy should not be initiated until a good “take” has occurred, which usually requires 3 to 4 weeks. In addition, a treatment schedule that employs a relatively large number of fractions, such as in Table 66-2, should be used. The entire graft should be included in the target volume (see the section on Field Size). In a study by Wilder and colleagues,144 all 21 basal cell carcinomas that extended to the surgical margin were controlled with radiation therapy. Immediate retreatment, either with surgery or radiation therapy, should be performed when a squamous cell carcinoma extends to the surgical margin because this type of cancer is more likely to recur locoregionally than basal cell carcinoma.
The data on adjuvant radiation therapy in the setting of perineural invasion is controversial.152 Because the presence of perineural invasion in patients with squamous cell carcinoma of the skin is often associated with aggressive clinical behavior, an increased risk of recurrence, and a poorer prognosis, postoperative radiation therapy is generally recommended. Among 135 patients treated at the University of Florida by surgery followed by postoperative radiation therapy, local control rates were 87% and 55% for those with microscopic and macroscopic perineural invasion, respectively.153 Although a combined-modality approach is generally preferred in this setting, the extent to which radiation therapy improved outcomes, particularly among those with microscopic perineural invasion, remains to be debated. When perineural invasion is suspected for patients with disease involving the head and neck, MRI of the base of skull and regional lymph nodes should be considered to fully evaluate the extent of possible spread and to assess the status of the regional lymph nodes.
Radiation also plays an important role in the management of parotid area metastasis from cutaneous skin cancer. Because the lymph nodes in and around the parotid gland receive most of the lymphatic drainage from the facial skin and scalp, they are the site most commonly involved when skin cancer from the head and neck metastasizes to the regional lymph nodes. Taylor and colleagues reported local control rates of 63% among patients treated with surgery alone compared with 89% for patients treated by surgery and postoperative radiation therapy.154 Another study from the University of California, San Francisco, demonstrated that inclusion of the ipsilateral N0 neck decreased the incidence of subsequent nodal failures from 50% to 0%.155
Techniques of Radiotherapy
Energy and Filtration
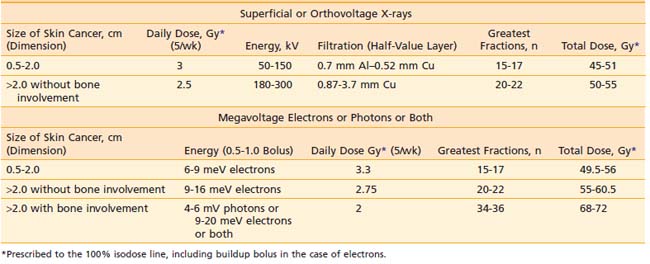
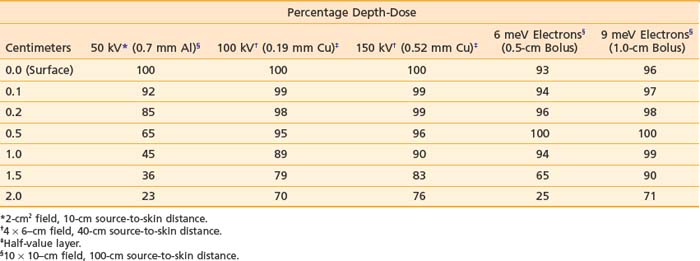
Orthovoltage x-rays and electron beams are most commonly used in the treatment of skin cancer. The technique used is determined by the size, depth, and anatomic location of the lesion. The quality of the radiation should be selected based on a review of the depth-dose characteristics of the available modalities (Table 66-3).
Table 66-3 Central Axis Percentage Depth-Doses as a Function of Energy, Buildup Bolus Thickness, Filtration, Field Size, and Source-to-Skin Distance
Based on articles involving superficial (50 to 150 kV) and orthovoltage (160 to 300 kV) x-rays156 that were published in the 1950s and 1960s,157–159 a number of authors have suggested that radiation therapy is contraindicated for carcinomas involving bone or cartilage because of the excessive risk of osteoradionecrosis and radiochondritis. However, more recent literature indicates that properly fractionated radiation therapy can be used in these cases and, in fact, is often the treatment of choice with or without surgery because the risk of radiation-induced complications is low when careful attention is paid to technical factors.143,160
Most carcinomas of the skin can be cured with superficial or orthovoltage x-rays or megavoltage electrons. Advantages of superficial or orthovoltage x-rays are that they require less of a margin on the skin surface around a carcinoma and are less expensive than electrons. In addition, when carcinomas of the eyelid are treated with orthovoltage x-rays rather than 6 meV electrons, the cornea receives a lower dose of radiation.161
Because irradiation of deep subcutaneous tissue increases the likelihood of conspicuous scarring, energy selection is important. The most commonly used peak superficial and orthovoltage x-ray energies are 50, 100, 150, 200, 250, and 300 kV. With superficial and orthovoltage x-rays (and megavoltage electrons), an energy should be selected such that the 90% depth-dose encompasses the target volume (see Table 66-3). Because most basal cell carcinomas extend to a depth of 2 to 5 mm,162 50-kV x-rays have been recommended in the dermatology literature. As presented in Table 66-4, a typical 50-kV beam delivers 85% of the surface dose at a depth of 2 mm and only 65% of the surface dose at a depth of 5 mm. Consequently, soft x-ray (50-kV) beams should be used only with very superficial lesions such as small eyelid tumors.163
Table 66-4 Five-Year Local Control Rates for Previously Treated Basal Cell Carcinoma
| 5-Year Local Control, % Treatment Modality | (Controlled/Treated, n/n) |
|---|---|
| Mohs micrographic surgery | 94 (2841/3009) |
| Radiation therapy | 87 (204/234) |
| Cryotherapy | —* |
| Surgical excision | 83 (431/522) |
| Curettage and electrodesiccation | 60 (69/115) |
* The 5-year results have not been published for cryotherapy. The weighted mean of the local control rates for studies with fewer than 5 years of follow-up is 87% (227/261).
Data from Rowe DE, Carroll RJ, Day CL Jr: Mohs’ surgery is the treatment of choice for recurrent (previously treated) basal cell carcinomas, J Dermatol Surg Oncol 15:424, 1989.
The roentgen-to-rad conversion (f) factor, relates exposure in air to the absorbed dose of radiation in tissue.156 The f factor depends on both radiation energy and tissue composition. For a tissue with an atomic number much greater than that of air, the f factor changes significantly as the radiation energy decreases to less than 300 kV (e.g., for compact bone, f factors equal 0.94 at 4 mV and 300 kV compared with 1.45 at 100 kV, whereas for skin, f factors equal 0.96 at 4 mV and 300 kV compared with 0.94 at 100 kV164). At energies below 300 kV, the dose of radiation in a tissue with a high atomic number (e.g., compact bone) is greater than the dose in a tissue with a low atomic number (e.g., skin). As a result, if a carcinoma invades bone, electrons or megavoltage photons or both provide a more homogeneous dose of radiation in the target volume than superficial or orthovoltage x-rays.
Filtration “hardens” a superficial or orthovoltage x-ray beam, meaning it removes low-energy x-rays,156
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree