41 Cancer of the Rectum
The sections in this chapter on epidemiology and genetics, pathologic conditions, routes of spread, and diagnostic and staging studies apply to both colon cancer and rectal cancer and are discussed in Chapter 40 on colon cancer. This chapter focuses exclusively on rectal cancer.
Epidemiology, Etiology, Genetics, and Cytogenetic Abnormalities
Information on epidemiology and genetics is covered in Chapter 40. In 2007 there were an estimated 41,420 new cases of rectal cancer in the United States with 23,840 occurring in men and 17,580 occurring in women.1
Anatomy
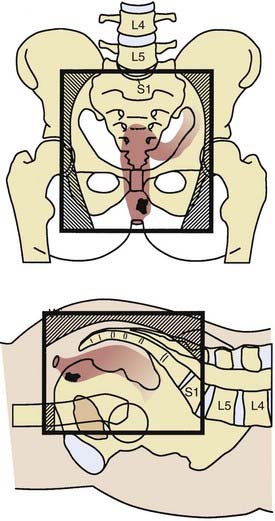
The rectum is approximately 15 cm in length. As seen in Fig. 41-1, it is divided into three 5-cm segments in relation to the anal verge (upper third, middle third, and lower third). However, the actual rectal length and division into surgical segments reflect a variety of features such as height, body habitus, pelvic width, and curve of the sacral hollow. For treatment purposes, large-bowel cancers that are located at or below the peritoneal reflection (rectosigmoid plus rectum) are collectively defined as rectal cancer.
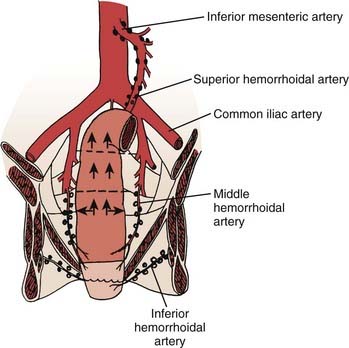
FIGURE 41-1 • Anatomy and lymphatic drainage of the rectum.
(From Pemberton JH: Anatomy and physiology of the anus and rectum. In Zuidema GD, ed. Shackelford’s Surgery of the Alimentary Tract. ed 2, Philadelphia, 1991, WB, Saunders, p 253.)
The major portion of the lymphatic drainage of the rectum passes along the superior hemorrhoidal arterial trunk toward the inferior mesenteric artery.2 Only a few lymphatics follow the inferior mesenteric vein. The pararectal nodes above the level of the middle rectal-valve drain exclusively along the superior hemorrhoidal lymphatic chain. Below this level (approximately 7-8 cm above the anal verge), some lymphatics pass to the lateral rectal pedicle. These lymphatics are associated with nodes along the middle hemorrhoidal artery, obturator fossa, and the hypogastric and common iliac arteries. Extensive lymphatics are also present in women contiguous with the rectovaginal septum, and in men along Denonvilliers fascia. The entire extraperitoneal soft tissue (mesorectum) is permeated with lymphatics.
Pathology
The majority of this information is covered in Chapter 40. However, the issue of lateral margins, which is most relevant to rectal cancer, is discussed.
In general, when pathologists describe negative margins of resection, they are referring to the proximal and distal margins. Because the staging of rectal cancer depends on the penetration of tumor through the bowel wall, the lateral (or circumferential or radial) margin is of equal importance. Birbeck and associates reviewed surgical specimens of 586 patients and found that 28% had a positive circumferential margin.3 There was a significant increase in local recurrence (38% versus 10%) and decrease in 5-year survival (40% versus 79%) in patients with positive versus negative circumferential margins. In a retrospective analysis of 504 patients reported by Bail and colleagues, even following preoperative combined-modality therapy (CMT), those with positive radial margins still had a higher local recurrence rate (35% versus 11%) and lower 5-year survival (27% versus 73%) compared with those with negative radial margins.4
Diagnostic and Staging Studies
The standard work-up for rectal cancer is seen in Table 41-1. The primary imaging modalities to assess the extent of the primary tumor are computed tomography (CT), magnetic resonance imaging (MRI), and transrectal ultrasound. The overall accuracy in predicting T stage is approximately 50% to 90% with transrectal ultrasound5–7 and 50% to 70% with CT or MRI.8–10 Positron emission tomography (PET) may offer a higher accuracy compared with CT for both primary and metastatic disease.11,12 PET is also helpful in predicting patients who will achieve negative margins at surgery.13 Obtaining a preoperative carcinoembryonic antigen (CEA) level is recommended; however, there is insufficient evidence at this time for the routine use of molecular markers.14
Table 41-1 Workup for Rectal Cancer
The identification of positive lymph nodes is more difficult. The overall accuracy in detecting positive lymph nodes with these techniques is approximately 50%. The accuracy of MRI is similar to CT; however, MRI may be further enhanced with the use of external or endorectal coils.10 Both CT and MRI can identify lymph nodes that measure 1 cm or larger, although enlarged lymph nodes are not pathognomonic of tumor involvement. Furthermore, using a nodal clearing technique, Herrera and colleagues found that 78% of positive lymph nodes in rectal cancer occur most frequently in lymph nodes measuring less than 5 mm.15 MRI may be further enhanced with the use of superparamagnetic iron oxide particles.16 Likewise, the accuracy of endorectal ultrasound for the detection of involved perirectal lymph nodes may be augmented if combined with fine-needle aspiration.7 The ability to accurately predict pathologic stage following preoperative CMT with MRI,17,18 ultrasound,19,20 PET,18,21 or physical examination22 is suboptimal.
Staging System
The sixth edition of the American Joint Commission on Cancer (AJCC) tumor-node-metastasis (TNM) staging system, as well as details regarding the evolution of pathologic staging of rectal cancer, are discussed in Chapter 40. Because many patients with rectal cancer are treated with preoperative CMT, most investigators recommend the TNM staging system with the provision that it is based on clinical or radiographic findings (e.g., ultrasound T3 [uT3] or clinical T4 [cT4]). The sixth edition of the AJCC TNM staging system recommends the y prefix (ycTNM or ypathologic [yp]TNM) when the tumor is staged during or after radiation or chemotherapy.23
One change is particularly relevant to rectal cancer. As with colon cancer, stage group II is subdivided into IIA (T3 disease) and IIB (T4 disease). Stage group III is subdivided into IIIA (T1-2 N1 M0 disease), IIIB (T3-4 N1 M0 disease), and IIIB (Tany N2 M0 disease). The prognostic validity of this change was supported by both the pooled analysis of the Intergroup and the National Surgical Breast and Bowel Project (NSABP) postoperative trials24 and the retrospective analysis of the American College of Surgeons National Cancer Database (NCDB).25 The 5-year survival by stages IIIA, IIIB, and IIIC in the pooled analysis was 81%, 57%, and 49%; and in the NCDB database was 55%, 35%, and 25%, respectively.
Standard Therapeutic Approaches
There are two conventional treatments for clinically resectable rectal cancer. The first is surgery and, if the tumor is pT3 or N1-2, this is followed by postoperative CMT.26 The second is used if the tumor is uT3 or cT4, and consists of preoperative CMT followed by surgery and postoperative chemotherapy.27 Based on the improved local control, toxicity profile, and incidence of sphincter preservation in patients who received preoperative CMT compared with postoperative CMT in the German Chirugische Arbeitsgemeinschaft Onkologie/Arbeitsgemeinschaft Radioonkologie/Arbeitsgemeinschaft Internistische Onkologie (CAO/ARO/AIO) 94 trial, preoperative CMT for patients with cT3 or N+ disease is now the standard of care.28
In patients with recurrent or clinically unresectable disease, intraoperative radiation therapy (IORT) is added at the time of surgery.29–32 In selected patients preoperative CMT followed by local excision33,34 or a local excision with or without postoperative CMT has been used.35,36 Radiation therapy alone has been used for patients who refuse surgery or are medically inoperable.37,38 The selection and results of these approaches are discussed in the Outcomes section. The National Comprehensive Cancer Network has published a clinical practice guideline for rectal cancer based on multidisciplinary panels, which use both evidence from randomized trials as well as lower-level sources such as clinical experience.39
Techniques of Radiation Therapy
The biologic mechanisms of acute and delayed toxicity as well as dietary interventions have been well described.40 Acute complications such as diarrhea and increased bowel frequency (small bowel), acute proctitis (large bowel), thrombocytopenia, leukopenia, and dysuria are common during treatment. These conditions are usually transient and resolve within a few weeks following the completion of radiation. The symptoms are related more to the dose rate and fraction size than the total dose. The mechanism is primarily the depletion of actively dividing cells in what is otherwise a stable cell-renewal system. In the small bowel, loss of the mucosal cells results in malabsorption of various substances, including fat, carbohydrate, protein, and bile salts. The bowel mucosa usually recovers completely in 1 to 3 months following radiation. Management usually involves the use of antispasmodic and anticholinergic medications.
The most common delayed severe complications are due to small-bowel damage and include small-bowel enteritis, adhesions, and small-bowel obstruction requiring surgical intervention. In the Massachusetts General Hospital (MGH) series, the incidence of small-bowel obestruction (SBO) with postoperative radiation therapy was 6% as compared with 5% with surgery alone.41
Preoperative radiation causes less acute and chronic toxicity compared with the postoperative treatment.28,42 This is likely the result of the fact that small bowel in an unviolated abdomen will be mobile and less likely to be adherent within a pelvic radiation portal. In the German CAO/ARO/AIO-94 trial, grade 3+ gastrointestinal toxicity was significantly reduced with the preoperative approach (acute 12% versus 18%, p = 0.04; and long-term 9% versus 15%, p = 0.07).28 Strictures at the anastomotic site were reduced 4% versus 12% (p = 0.003). The incidence of SBO requiring surgery in the preoperative arm of the German trial was 2%.
Toxicity of Pelvic Radiation Therapy
Complications of pelvic radiation therapy are a function of the volume of the radiation field, overall treatment time, fraction size, radiation energy, total dose, and technique. A number of simple radiotherapeutic techniques are available to decrease radiation-related small-bowel toxicity when using conventional techniques (Table 41-2).43,44 For example, the use of multiple-field techniques (preferably a three-field technique) allows a larger amount of small bowel to be blocked from the pelvis. The treatment of all fields each day results in a lower integral dose and more homogeneous dose distribution. The use of lateral fields for the boost, as well as positioning the patient in the prone position, further decreases the volume of small bowel in the lateral radiation fields. The treatment should be delivered by high-energy linear accelerators (>6 mV), which, by nature of their depth-dose characteristics, deliver a higher dose to the tumor volume while sparing the surrounding normal structures. When the perineal scar needs to be treated, it should be included in the pelvic radiation fields. The use of a separate perineal field is associated with an increased risk of overlap of the radiation fields and should be avoided. Split-course pelvic radiation is associated with increased chronic bowel complications.45
Table 41-2 Techniques to Decrease Radiation Toxicity in Small Bowel
Active inflammatory bowel disease is a contraindication to pelvic radiation, although there are some reports of patients who have tolerated it.46,47 Pelvic fractures following pelvic radiation are rare.48,49 Even with appropriate doses and techniques of radiation, approximately 1% of patients have significant long-term toxicity to pelvic organs. Testosterone levels are decreased when the testicles are near or in the radiation field.50,51 Radiation, alone or in combination with surgery, can have a negative effect on sexual function.52–54
Small-Bowel Contrast
The small bowel is a dose-limiting organ,55 and complications are proportional to the volume of small bowel in the radiation field.56 Small-bowel contrast is essential to determine the position of the small bowel during simulation. It should be used routinely during the simulation in patients receiving curative pelvic radiation therapy. Nonfixed small bowel normally moves, and a CT scan may be more accurate than traditional small-bowel series in detecting the position and volume of small bowel.57
Physical Maneuvers
Various physical maneuvers to exclude the small bowel from the pelvis have been examined. Gallagher and colleagues reported a significant decrease in the average small-bowel volume when the patients were treated in the prone position with the combination of abdominal-wall compression and bladder distension compared with the supine position.58 Use of a four-field technique further decreased the volume of small bowel. Treatment in the prone position without abdominal wall compression was not consistently effective in displacing small bowel and, in some patients, most commonly obese, the volume of small bowel increased.
Using a three-dimensional (3-D) planning system, Koelbl and associates found that in patients who received postoperative radiation, the use of the prone position plus a belly board decreased the small-bowel volume treated versus the use of the supine position.59 Intensity-modulated radiation therapy (IMRT) treatment-planning techniques can further decrease the volume of small bowel in the field.60
Immobilization Molds
The effectiveness of custom bowel immobilization molds (belly board) have been documented in a number of series.61,62 Shanahan and colleagues reported that the combination of the prone position and immobilization molds decreased the mean small-bowel volume in the radiation field by 66% compared with patients treated in the supine position without the immobilization mold.61
Any physical maneuver beyond the use of the prone position may be associated with patient discomfort, thereby leading to increased movement and daily set-up errors. Brierley and colleagues analyzed the variation of small-bowel volume in the pelvis before and during adjuvant pelvic radiation therapy for rectal cancer and reported that the displacement of small bowel from the posterior pelvis by bladder distension was not reliably maintained throughout the treatment course.63 Therefore, physical maneuvers beyond the prone position may not be beneficial in all patients. The use of such techniques should be tailored to the individual patient.
Radioprotectors and Radiosensitizers
Randomized trials have investigated the use of sucralfate enemas to decrease acute radiation proctitis, olsalazine and mesalazine to decrease acute enteritis, and butyric acid to decrease chronic radiation proctitis.64–68 All of these trials have been negative. The radioprotector WR-2721 did not reduce toxicity in early trials, but there is a suggestion of a benefit in a more recent study.69 Rectal-administered amifostine is well tolerated; however, its efficacy remains to be determined.70,71
Altered Radiation Fractionation Schemes
Hyperfractionated radiation has been examined in phase I and II trials.72,73 In general, the pathologic complete response (pCR) rates may be improved, but at the expense of increased acute toxicity. In the series by Janjan and associates, the use of a concomitant boost during standard CMT increased the incidence of perioperative morbidity.74 Movsas and colleagues recommend limiting the twice-daily component to the boost,75,76 whereas Myerson and colleagues deliver a concomitant 3-D boost one or twice a week.77 The Radiation Therapy Oncology Group R-0012 phase II randomized trial compared preoperative CMT with twice-daily radiation up to 60 Gy (1.2 Gy to 45.6 Gy, with a boost of 9.6-14.4 Gy) with conventional fractionation (1.8 Gy to 45 Gy, with a boost of 5.4-9.0 Gy) plus 5-fluorouracil (5-FU)/irinotecan.78 Both regimens resulted in a 28% pCR rate, but were also associated with a more than 40% rate of grade 3 or 4 acute toxicity. Hyperfractionated and accelerated fractionated radiation, especially in combination with chemotherapy, remains investigational.
Three-Dimensional Treatment Planning and Intensity-Modulated Radiation Therapy
Innovative techniques using 3-D treatment planning are being investigated.79 The most important contributions of 3-D treatment planning are the ability to plan and localize the target and normal tissues at all levels of the treatment volume, and to obtain dose-volume histogram data. A randomized trial of conformal versus conventional radiation therapy in 266 evaluable patients with pelvic malignancies has been reported by Tait and colleagues.80 Although there was a decrease in the volume of normal tissue volumes in the radiation field with conformal versus conventional treatment (689 cm3 versus 792 cm3), there was no difference in the level of symptoms or in medication prescribed. Meyerson and associates used a 3-D planned boost radiotherapy (0.9 Gy once or twice weekly to a total boost dose of 4.5 to 9 Gy) concurrently with pelvic irradiation (45 Gy/25 fractions).77 Dose-volume histogram information correlated with grade 3 and 4 toxicity, particularly with respect to small-bowel complications. The authors concluded that every effort should be made to limit the volume of small bowel receiving more then 40 Gy to less than 120 cc. Using a 3-D planning system, Koelbl and associates found that in patients receiving postoperative radiation, the use of the prone position plus a belly board decreased the small-bowel volume treated versus the supine position.81 An analysis of 3-D treatment planning techniques at the MGH suggests that the volume of small bowel in the radiation field is decreased with protons as compared with photons.82
To limit dose to previously irradiated critical structures, 3-D planning techniques are desirable for patients who undergo reirradiation. Although not well studied to date, the use of IMRT may further lower the dose to the critical structures while maintaining adequate doses in the planning target volume.83,84
Other Investigational Approaches
Investigational approaches such as neutrons,85 carbon ions,86 and hyperthermia87–89 have been examined. None have shown a clear advantage compared with conventional pelvic radiation therapy.
Surgical Techniques
Surgical techniques to minimize small-bowel injury in the postoperative setting include placing clips in the high-risk areas to better define the tumor volume and the use of absorbable mesh, which temporarily removes the small bowel from the pelvis.43 Because the radiation component of postoperative CMT does not begin until 4 months after surgery, the mesh may have already resorbed. Other techniques such as an inflatable pelvic small-bowel displacement prosthesis,90 reconstruction of the pelvic floor, construction of an omental pedicle flap,91 and retroversion of the uterus have had variable success.
Design of Radiation Therapy Fields for Rectal Cancer
The majority of local failures are in the posterior one half to two thirds of the true pelvis.43 Because the internal iliac and presacral nodes are posterior in reference to the external iliac nodes, much of the normal structures in the anterior pelvis can be spared with the use of lateral fields.
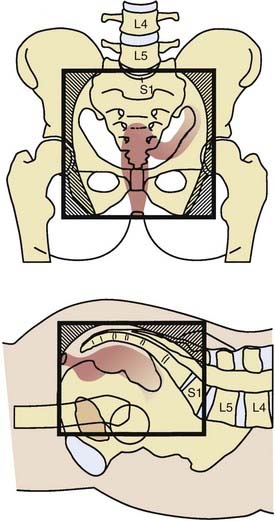
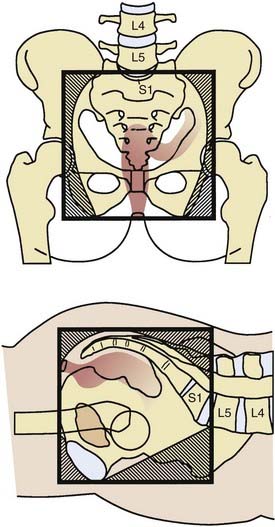
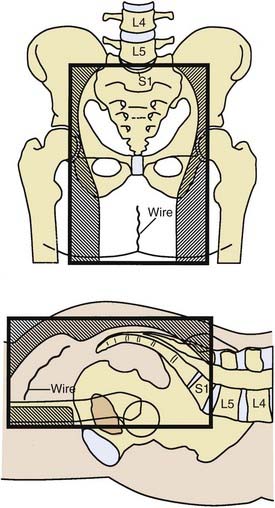
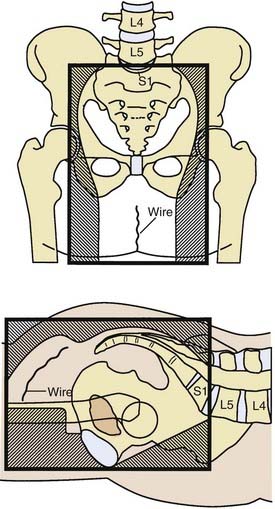
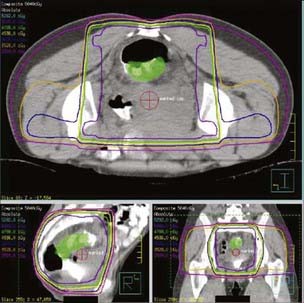
General guidelines for the design of conventional pelvic radiation therapy fields are listed in Table 41-3. The whole pelvic radiation field should adequately cover the primary bed as well as the primary nodes at risk. The intent of the boost is to treat the primary tumor and not to include the nodes. Therefore, the exact size is determined by the size and location of the primary tumor. Specific examples of field arrangements for a variety of clinical presentations are seen in Figs. 41-2 through Fig. 41-7. An example of a 3-D treatment plan is seen in Fig. 41-8.
Table 41-3 General Guidelines for Pelvic Radiation Therapy for Rectal Cancer
Outcome
Patterns of Failure
Local recurrence as a site of failure occurs frequently in patients with rectal cancer. The incidence is directly related to the extent of transmural penetration (microscopic versus gross) and the number and location of positive lymph nodes.92 The most common metastatic site is liver followed by lung. Brain93 and inguinal node94 metastases are uncommon. As more effective systemic chemotherapy is used, resulting in median survivals of up to 24 months in patients with metastatec disease,95,96 there is an increase in metastases seen in both these as well as other uncommon sites.
Clinically Resectable Rectal Cancer
Preoperative Radiation
A meta-analysis concluded that biologically effective preoperative doses of more than 30 Gy resulted in a statistically significant reduction in local-regional recurrences.97 With conventional fractionation (1.8-2.0 Gy fractions, 5 days per week), the doses most commonly used for the whole-pelvis fields, either preoperative or postoperative, range from 45 to 50.4 Gy in 5 to 6 weeks. These doses are necessary to control microscopic disease.98 An additional boost of 5.4 Gy to the primary tumor or tumor bed may be delivered if the small bowel is excluded from the high-dose field. However, it is not clear that higher doses improve local control. Doses up to 60 Gy are associated with increased pCR rates; however, they may also significantly increase acute and long-term morbidity.
In the Lyon R96-02 trial, 88 patients with cT2-3 rectal cancer received 39 Gy in 13 fractions preoperatively to the pelvis and were randomized to observation or a boost with contact radiation to a total dose of 85 Gy.99 Patients who received the boost had a decrease in local failure (2% versus 7%), but no difference in 2-year disease-free survival (92% versus 88%).
A unique clinical situation in which preoperative radiation therapy alone is recommended is when a patient has a distal uT2-N0 tumor and refuses an abdominoperineal resection (APR). Although APR is the standard therapy, an alternative is treatment with preoperative radiation followed by low anterior resection (LAR) and, if the pelvic nodes are positive, postoperative chemotherapy. In a series of 27 patients with cT2 N0 distal rectal cancer who refused APR, 78% of patients underwent a sphincter-sparing operation.100 The incidence of 5-year actuarial local recurrence of those undergoing sphincter preservation was 13%, colostomy-free survival was 100%, and overall survival was 85%. Overall, 77% of those undergoing a sphincter-sparing procedure had good or excellent bowel function at 24 to 36 months after surgery.
Short-Course Preoperative Radiation
There are 12 modern randomized trials of preoperative radiation therapy (without chemotherapy).27 All use low to moderate doses of radiation. Most of the trials showed a decrease in local recurrence, and in five of the trials, this difference reached statistical significance. Although in some trials a subset analysis revealed a significant improvement in survival, the Swedish Rectal Cancer Trial is the only one that reported a survival advantage for the total treatment group. Two meta-analyses report conflicting results. Although both revealed a decrease in local recurrence, the analysis by Camma and colleagues101 reported a survival advantage, whereas the analysis by the Colorectal Cancer Collaborative Group97 did not.
In the Swedish Rectal Cancer Trial, patients with cT1-3 rectal cancer were randomized to receive 25 Gy in 1 week followed by surgery 1 week later, versus surgery alone.102 Those who received preoperative radiation had a significant decrease in local recurrence (12% versus 27%) and a corresponding significant improvement in 5-year survival (58% versus 48%), with 13-year follow-up survival still significantly improved (38% versus 30%, p = 0.008).103 Of interest, the local recurrence rate in lymph node–positive (LN+) patients who underwent surgery alone was 46%, illustrating the inferior results of surgery prior to the adoption of total mesorectal excision (TME).
The Dutch CKVO 95-04 trial randomized 1805 patients with cT1-3 disease to TME or short-course preoperative radiation followed by TME.104 Although radiation significantly decreased local recurrence (8% versus 2%), there was no difference in 2-year survival (82%). With longer follow-up, 5-year local failure was higher with TME (11%); however, it was still significantly decreased to 6% with preoperative radiation.105 The acute toxicity in the Dutch CKVO 95-04 trial included 10% neurotoxicity, 29% perineal wound complications, and 12% postoperative leaks.106 In the patients who developed postoperative leaks, 80% required surgery resulting in 11% mortality.
The presence of a positive circumferential margin is an important negative prognostic sign. In the Dutch CKVO trial, 17% had positive circumferential margins. Although they received 50 Gy postoperatively, this did not compensate for positive margins.107 Few centers perform the necessary pathologic examination to detect positive circumferential margins.4,108 MRI can help identify patients who will have positive margins with surgery alone, as well as select those who may benefit from preoperative therapy.109–111
Combined Modality Therapy
There are two conventional treatments for clinically resectable rectal cancer. The first is surgery and, if the tumor is pT3 or N1-2, this is followed by postoperative CMT.26 The second is preoperative CMT followed by surgery and postoperative CMT if the tumor is uT3-4 or N+.27
Postoperative Therapy
The National Cancer Institute (NCI) Consensus Conference concluded in 1990 that CMT was the standard postoperative adjuvant treatment for patients with pT3 or N1-2 disease.26 Pelvic radiation therapy decreased local recurrence by approximately 50%, but did not improve survival. The standard design is to deliver six cycles of chemotherapy, two of which are given with concurrent radiation during the third and fourth cycles.
For patients treated with postoperative CMT receiving 5-FU as a single agent, there was a 10% survival advantage with continuous infusion (CI) 5-FU versus bolus 5-FU.112 The intergroup trial (INT) 0144 postoperative adjuvant rectal trial did not confirm this survival benefit; however, it did report a lower incidence of grade 3+ hematologic toxicity.113 Given these results, when 5-FU is used with either preoperative or postoperative CMT, CI is the standard. Although it is just now being studied in rectal cancer, the combination of CI–5-FU and oxaliplatin (FOLFOX) has replaced CI–5-FU as a standard postoperative chemotherapy treatment based on the efficacy demonstrated in stage III colon cancer patients.114
The INT 0114 4-arm trial randomized patients with pT3 or N+ rectal cancer to postoperative radiation and bolus 5-FU with or without leucovorin, levamisole, or leucovorin plus levamisole. There was no significant difference in local control or survival among the four arms.115 With longer follow-up, the study also revealed that local control and survival results continue to decrease after 5 years. At 7 years, local failure rate was 17% and the survival was 56% compared with 14% and 64%, respectively, at 5 years. Patients with high-risk (pT3 N+ or T4) disease had a lower survival compared with lower risk (pT1-2 N+ or T3 N0) disease (45% versus 70%). Further analysis of the INT 0114 trial has revealed that body mass is related to outcome and treatment-related toxicity,116 and both surgeons and hospitals with higher volumes of rectal cancer surgery have improved outcomes compared with those with lower volumes.117
Almost every randomized trial for the past 2 decades has confirmed a 10% to 15% survival benefit following 6 months of adjuvant 5-FU based chemotherapy for patients with LN+ colon or rectal cancer. One retrospective118 and two randomized trials119,120 question whether chemotherapy improves the results of preoperative radiation in patients with cT3 rectal cancer. The European Organisation for Research and Treatment of Cancer (EORTC) 22921 trial was a four-arm randomized trial of 1011 patients who received preoperative 45 Gy with or without concurrent bolus 5-FU/leucovorin followed by surgery with or without four cycles of postoperative 5-FU/leucovorin.121 Only 37% had a TME. The Fédération Francophone de Cancérologie Digestive (FFCD) 9203 trial was a two-arm randomized trial of 742 patients randomized to preoperative 45 Gy with or without bolus 5-FU/leucovorin.122 However, all patients were scheduled to receive postoperative chemotherapy, and 73% did.
The EORTC trial revealed a significant decrease in the local failure rate in those patients who receive CMT compared with radiation (8%-10% versus 17%, p < 0.001), but no difference in 5-year survival (65%). However, only 43% received 95% or more of the planned postoperative chemotherapy, which may explain the negative results. Furthermore, a subset analysis of the EORTC trial revealed that patients who responded to preoperative CMT had a survival benefit from postoperative chemotherapy.123
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree