50 Cancer of the Ovary
Epidemiology and Diagnosis
In 2009, ovarian cancer will be diagnosed in approximately 21,550 American women, and an estimated 14,600 will die of the disease, making it the fifth most common cancer in women and the most common cause of gynecologic cancer mortality.1 Approximately 1 in 70 women will develop ovarian cancer in their lifetime. Ovarian cancer is more common in northern European and North American countries than in Asia, developing countries, or southern continents.
The etiology of ovarian cancer is unknown, but risk factors have been identified (Table 50-1). Recognized risk factors for the disease include advancing age,2 with an annual incidence of 1 in 11,000 at age 40, 1 in 4500 at age 50, 1 in 2600 at age 60, and 1 in 2000 at age 70. Other factors associated with an increased risk include infertility, endometriosis, the use of assisted reproductive technologies, and application of perineal talc.3 The incremental risk for developing ovarian cancer associated with any of these age-independent risk factors is relatively small, with relative risks of no more than 2 to 3 compared to the general population. Smoking, alcohol use, coffee consumption, and viral infections (such as mumps) have not been associated with increased risk.
Table 50-1 Risk Factors for Ovarian Cancer
Approximately 8% to 13% of ovarian cancer is due to inherited mutations4,5 in the cancer susceptibility genes BRCA1 and BRCA. Women with mutations in the BRCA1 gene have a 35% to 60% risk for developing ovarian cancer by age 70, corresponding to a relative risk of approximately 30 to 45 compared to the general population.6 BRCA2 mutations are associated with a 10% to 27% risk of ovarian cancer by age 70, which corresponds to a relative risk of 6 to 20. Approximately 1% to 2% of ovarian cancers are associated with inherited defects in the DNA mismatch repair genes MLH1, MSH2, and MSH6, associated with the hereditary nonpolyposis colon cancer (HNPCC) syndrome.7 Women with mutations in these genes have a 9% to 12% risk of developing ovarian cancer by age 70, corresponding to a relative risk of 6 to 9.
In the absence of genetic testing information, a family history of ovarian cancer or early-onset breast cancer has been associated with an increased risk of ovarian cancer, with a relative risk of approximately 3 to 5 compared to the general population.8 It is not clear, however, how much of this increased risk is accounted for by mutations in the known ovarian cancer susceptibility genes.
In regard to protective factors, multiple studies have demonstrated that oral contraceptive use and parity are associated with a decreased risk of ovarian cancer.9 Use of oral contraceptives is protective for ovarian cancer, with an average relative risk of about 0.7 for women who have used oral contraceptives for 2 years and a 0.5 relative risk for women who have used oral contraceptives for 5 years or longer. A woman’s risk for ovarian cancer also may be reduced if she has had tubal sterilization, a hysterectomy, or at least one child whom she has breastfed.10
Screening
One of the most significant ways to improve survival for patients with ovarian cancer would be to find a way to screen women for ovarian cancer and detect the disease before it spreads beyond the ovary. Recommendations for ovarian cancer screening have traditionally been stratified according to level of risk, with different recommendations put forth for women at average risk compared to women at increased risk. With the identification of autosomal dominant ovarian cancer susceptibility syndromes, the increased risk group has been further stratified to a group with a clear inherited risk and a group with increased risk by virtue of family history but in the absence of a known inherited cancer predisposition (Table 50-2).
Table 50-2 Ovarian Cancer Risk Types
| Women At or Near the General Population Risk (Relative Risk Less Than 3) |
| Individuals at Increased Risk (Relative Risk of 3 to 5) |
A personal history of breast cancer prior to age 50 and one or more close relatives* with breast or ovarian cancer at any age |
| Individuals at Inherited Risk (Relative Risk Greater Than 5) |
HNPCC, Hereditary nonpolyposis colon cancer.
* A close relative is defined as a first-, second- or third-degree relative.
There have been a number of tests evaluated as potential methods for screening for ovarian cancer. Screening tests with the greatest evidence base include serum CA125 and transvaginal ultrasound. CA125, a high molecular-weight protein excreted by greater than 90% of advanced epithelial ovarian cancers, has been the most evaluated serum marker for ovarian cancer screening. In the largest study to date, 22,000 postmenopausal women at average risk of ovarian cancer were randomized to either annual serum CA125 or usual gynecologic care.11 Ultrasound was performed in women in the screening arm if the CA125 was greater than 30 U/mL. In this study, women with screen-detected ovarian cancer had improved survival compared to women diagnosed with ovarian cancer who were randomized to usual care. While these results were promising, there was no difference between the two groups in the number of deaths due to ovarian cancer. To improve the utility of CA125 measurements for ovarian cancer screening, a method has been proposed which focuses on the change in CA125 concentration over time as opposed to the absolute value.
In the largest study to date evaluating ultrasound as a screening method for ovarian cancer, 14,469 women predominantly at average risk for ovarian cancer were followed with annual transvaginal ultrasounds.13 In this study, ultrasound was associated with an 81% sensitivity and a 98.9% specificity, resulting in a positive predictive value of 9.4%. Several studies have evaluated the simultaneous use of transvaginal ultrasound and CA125. These studies have suggested that the combination of these tests result in a higher sensitivity for ovarian cancer detection, but at the cost of an increased rate of false-positive results. In the ongoing PLCO trial, 28,816 women were randomized to receive annual transvaginal ultrasound and CA125. At baseline, 1338 (4.7%) ultrasounds and 402 (1.4%) CA125s were abnormal. Workup of these abnormalities led to the diagnoses of 20 invasive ovarian cancers. The positive predictive values for an abnormal test were 1% for transvaginal ultrasound and 3.7% for CA125. This did, however, increase to 23.5% when both were abnormal.14
In 2002, a report from researchers at the National Cancer Institute suggested that by evaluating the low molecular protein spectrum in peripheral serum, one could identify a characteristic “proteomic” pattern that was both highly sensitive (100%) and specific (95%) for early ovarian cancer.15 The initial report was promising, but this approach has not been validated in a prospective screening study.
Numerous national organizations such as the American Cancer Society (ACS), the American Congress of Obstetricians and Gynecologists (ACOG), the Society of Gynecologic Oncologists (SGO), and the National Comprehensive Cancer Network (NCCN) have made ovarian cancer screening recommendations. Based on these recommendations, ovarian cancer screening guidelines stratified by ovarian cancer risk type are shown in Table 50-3.
Table 50-3 Ovarian Cancer Screening Guidelines
Histology
Ovarian tumors are usually categorized16 by their tissue of origin: epithelial (from the coelomic epithelial cells that line the ovary), germ cell (from the germinal epithelium), and sex cord–stromal (from the mesenchymal tissue of the ovary). Epithelial tumors account for approximately 85% of ovarian cancers, germ cell cancers account for approximately 10%, and the remaining 5% of tumors are sex cord–stromal (Table 50-4).
Table 50-4 Histologic Classification of Common Epithelial Tumors of the Ovary
| Serous Tumors |
| Mucinous Tumors |
| Endometrioid Tumors |
| Clear Cell Tumors |
| Squamous Cell Carcinoma |
| Mixed Epithelial Tumors (specify types) |
Modified from Ozols RF, Rubin SC, Thomas GM, et al: Epithelial ovarian cancer. In Hoskins WJ, Perez CA, Young RC, et al, editors: Principles and practice of gynecologic oncology, ed 4, Philadelphia, 2005, Lippincott Williams & Wilkins, pp 895–987.
Of the malignant epithelial ovarian cancers, 40% to 50% are serous tumors, 15% to 25% are endometrioid tumors, 6% to 16% are mucinous tumors, and 5% to 11% are clear cell tumors (Fig. 50-1). Transitional cell (Brenner), mixed epithelial, and undifferentiated carcinomas are encountered less frequently. Epithelial tumors may be benign, have low malignant potential, or be frankly malignant. Tumors of low malignant potential (borderline tumors) have a much better prognosis than the frankly malignant cancers, but they are nevertheless malignant and can result in death. Malignant tumors are further subdivided by histologic grade into either three grades based on architecture (International Federation of Gynecology and Obstetrics [FIGO] classification) or four grades based on nuclear atypia (Broders’ index).
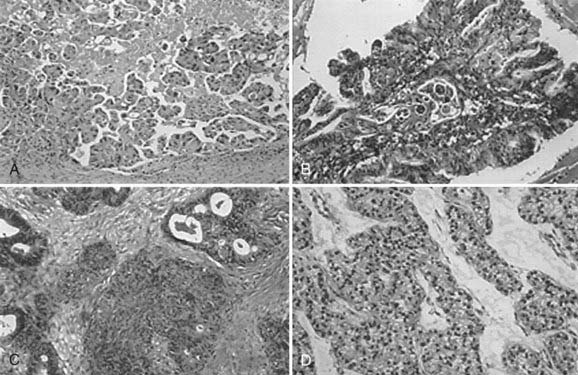
FIGURE 50-1 • Serous papillary (A), mutinous (B), endometriosis (C), and clear cell (D) tumors of the ovary (H&E stain, magnified ¥400).
Molecular Biology
In addition to abnormalities in BRCA1 or BRCA2 and DNA mismatch repair genes, advances in the molecular biology of ovarian cancer have shed some light on its pathogenesis, classification, and prognosis.17,18 The TP53 gene located on chromosome 17p belongs to the family of tumor-suppressor genes, and mutations in these genes have been implicated in malignant transformation and tumor progression. Mutation and loss of TP53 function is the most common genetic abnormality in ovarian cancer, especially high-grade serous cancer, and is seen in 60% to 80% of both sporadic and familial cases. There seems to be a correlation between the frequency of mutation in p53 and the stage of ovarian cancer (4% of preinvasive borderline, 10% to 20% of early cancers, and 40% to 60% of advanced cancers) and spread.19–21 HER2-neu belongs to the epidermal growth factor (EGF) receptor gene family and is a potent proto-oncogene. HER2-neu is overexpressed in approximately 15% to 20% of ovarian cancers, especially mucinous. Berchuck et al.22 reported that patients with increased HER2-neu expression had a survival median of 15.7 months, compared with 32.8 months in patients with normal neu expression. Mutations in K-ras, also a proto-oncogene, is seen in about 25% to 50% of mucinous ovarian cancer and most low-grade serous tumors.18
Diagnosis
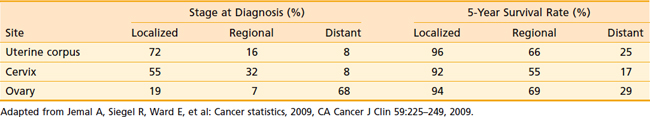
Table 50-5 illustrates the relative stage at diagnosis and the survival rate by stage for ovarian cancer in relation to the two other major gynecologic cancers. The 5-year survival rate for ovarian cancer by stage is not significantly different from the 5-year survival rate for other gynecologic cancers. However, at the time of diagnosis, two-thirds of patients with ovarian cancer have advanced-stage disease, unlike uterine corpus and cervical cancer, which are generally diagnosed in earlier stages. It is clear from these data that the single most important factor in the large number of deaths from ovarian cancer is failure to diagnose the disease at an early stage. Reasons for this failure correspond to the growth and spread patterns of this cancer. Because the ovary floats freely in the pelvic cavity, a tumor may grow for some time while producing only vague symptoms associated with involvement of, or pressure on, other organs. Thus ovarian cancer is diagnosed in most patients after the disease has spread beyond the ovary. In these patients, symptoms may be abdominal pain or a bloated feeling, gastrointestinal or urinary tract disturbances, or in many cases, the onset of clinically detectable ascites. Some patients with advanced disease have menstrual irregularity or postmenopausal bleeding. Occasionally a patient may have a palpable inguinal lymph node, tumor in a hernia sac, or a pleural effusion. For patients with advanced disease, diagnosis is established by tissue obtained at exploratory laparotomy. In rare instances when a patient cannot undergo surgery because of medical problems, the histologic or cytologic diagnosis is established by needle biopsy.
Table 50-5 Stage at Diagnosis and 5-Year Survival Rate of Gynecologic Cancers, United States, 1995-2001
Studies have shown that women with ovarian cancers, even women with early-stage disease, often experience symptoms several months before the diagnosis. In a survey of 1725 women with ovarian cancer, 70% recalled having had symptoms for 3 months or longer before the diagnosis, and 35% recalled having had symptoms for at least 6 months.23 About three-fourths of these women had abdominal symptoms, and half had pain or constitutional symptoms. Persistent symptoms such as an increase in abdominal size, abdominal bloating, fatigue, abdominal pain, indigestion, inability to eat normally, urinary frequency, pelvic pain, constipation, back pain, urinary incontinence of recent onset, or unexplained weight loss should be evaluated, with ovarian cancer being included in the differential diagnosis.
The use of tumor markers to assist in evaluation of a patient with an adnexal mass is appropriate, but misinformation may result. Serum CA125 is the only serum marker available with the potential accuracy to be beneficial, but even this marker is less than optimal. Approximately half of patients with early-stage ovarian and other cancers do not have elevated serum CA125 levels. Also, a variety of nonmalignant and nonovarian malignant conditions can result in elevated serum CA125 levels. These conditions are listed in Table 50-6. Computed tomography (CT) and magnetic resonance imaging (MRI) provide useful diagnostic and staging information, especially in delineating abdominal and pelvic masses and diagnosing retroperitoneal nodal involvement. The role of positron emission tomography (PET) is under investigation. In one study, the addition of PET to CT improved the staging accuracy from 85% to 93% outside the pelvis and from 79% to 81% in the pelvis.24 However, none of the procedures are sufficient enough for accurate staging, and laparotomy remains the single most important method for assessing the stage of disease and the design of treatment.
Table 50-6 Conditions That May Cause Elevated Serum CA125 Levels
| Malignant Conditions |
| Benign Conditions |
Staging
The FIGO staging classification scheme for ovarian cancer25 is outlined in Table 50-7. The staging of advanced disease (disease spread throughout the abdomen) may be obvious to most physicians, but a surgeon must be meticulous in the staging of early ovarian cancer. One study found that a third of patients referred with stage I or stage II disease were found to actually have stage III disease when the appropriate staging procedure was performed. Other researchers have reported similar results.
Table 50-7 FIGO Staging Classification for Ovarion Cancer
Rights were not granted to include this table in electronic media. Please refer to the printed book.

Table 50-8 outlines the recommended surgical staging procedures used for ovarian carcinoma that appears to be confined to the pelvis. The appropriate performance of a staging operation requires an understanding of the spread patterns of the cancer. Ovarian cancer can spread by direct infiltration of pelvic structures such as the pelvic peritoneum, bladder surface, rectal surface, fallopian tube, broad ligament, or uterus. Lymphatic spread occurs early, with nodal metastases present in 10% to 12% of patients with stage I cancer and 20% to 25% of patients with stage II disease. In stage III and stage IV, the incidence of positive lymph nodes is 50% to 70%. By far the most significant spread of ovarian cancer, however, is due to exfoliation of clonogenic cells into the peritoneal cavity. These cells are swept up the right abdominal gutter to the diaphragm and omentum by the clockwise flow of peritoneal fluid in the abdomen. The cells implant, form tumor nodules, and in turn exfoliate more cells. The normal daily activities of the patient and normal peristalsis of the intestine can result in spread of the disease throughout the abdominal cavity. Proper staging requires a generous lower and upper midline incision and meticulous exploration with multiple peritoneal and nodal biopsies.
Table 50-8 Diagnostic Procedures in the Diagnosis and Staging of Patients With Ovarian Carcinoma
Treatment of Primary Epithelial Ovarian Cancer
The treatment of epithelial ovarian cancer usually involves several types of therapy. Surgical therapy is the initial form of intervention, but it is curative in only a small percentage of cases. Usually, adjunctive chemotherapy is necessary. In a large percentage of patients, some type of salvage therapy is important. Table 50-9 outlines current therapeutic recommendations for epithelial ovarian cancer. The role of radiation in the management of ovarian carcinoma continues to represent a topic of considerable controversy, and the indications for its use are not fully established.
Table 50-9 Recommended Therapy for Epithelial Ovarian Cancer
| Category of Ovarian Cancer | Recommended (Standard) Therapy |
|---|---|
| Early Ovarian Cancer | |
| Low risk (stages IA and IB, grade 1*) | TAH, BSO†, full surgical staging |
| High risk (stages IA and IB, grades 2 and 3; stages IC, IIA, IIB, and IIC, no residual) | |
| Advanced Ovarian Cancer | |
| Stage III with optimal residual disease‡ | |
| Stage IV and/or suboptimal§ | |