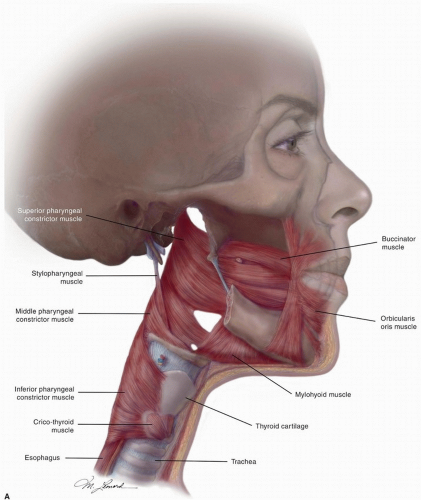
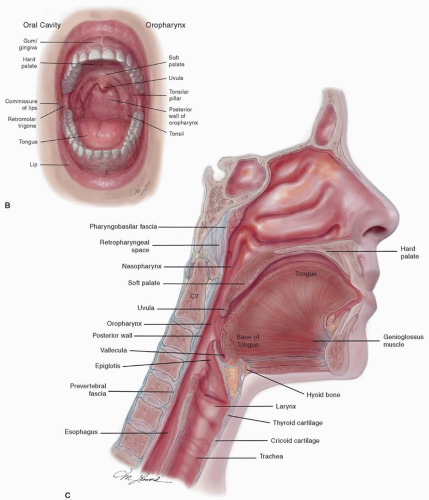
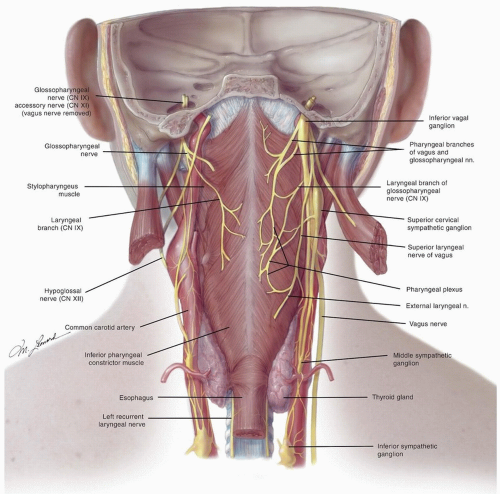
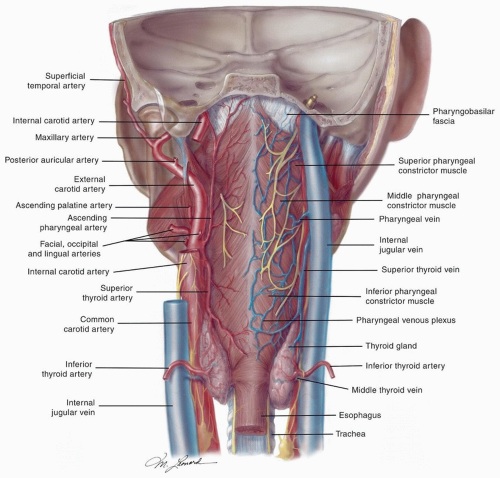
posterior belly of the digastric. The posterior system can also drain bilaterally to the retropharyngeal nodes via penetration of the lymphatics through the superior constrictor muscles.6
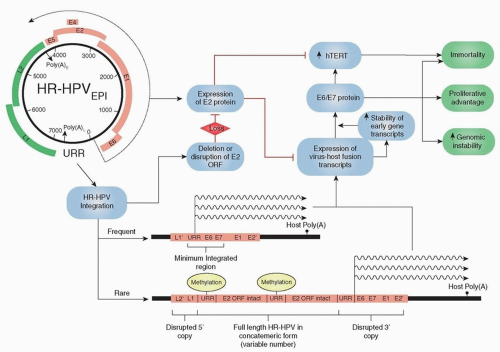
have been isolated, with low-risk types (e.g., HPV 6, 11) inducing benign hyperproliferation of the epithelium, leading to lesions such as papillomas and warts. High-risk types (e.g., HPV 16, 18, 31, 33, 35) are defined by epidemiologic associations with cervical cancer.19 The prototypical oncogenic types 16 and 18 account for over 90% of HPV-related OPSCC and are capable of malignant transformation of primary human keratinocytes from either genital or upper respiratory tract epithelia.20 This transforming potential is attributed to two HPV oncoproteins, E6 and E7, that inactivate two human tumor-suppressor proteins, p53 and pRb, respectively. These viral oncoproteins lead to transformation through the stimulation of cellular proliferation, delay of cellular differentiation, increase in the frequency of spontaneous and mutagen-induced mutations, and promotion of focal and broad chromosomal instability in transfected cell lines21 (Fig. 14.5). Furthermore, in order to maintain a malignant phenotype, a transcriptionally active viral genome appears to be necessary.22,23,24,25
The spindle cell component tends to form the bulk of the tumor with the SCC being restricted to the stalk or base. These cancers will stain for both epithelial markers including keratin and mesenchymal markers including vimentin.39
majority of minor salivary gland tumors arise in the base of the tongue, followed by the soft palate and palatine tonsil.46 The histologic heterogeneity in the minor salivary glands mirrors that of the major salivary glands, with adenoid cystic and mucoepidermoid carcinomas being the most frequent. There are conflicting data as to which neoplasm is actually the most frequent, but a recent series that focused on minor salivary gland malignancies of the oropharynx identified mucoepidermoid carcinomas as the most frequent, followed by adenoid cystic carcinoma, polymorphous low-grade adenocarcinoma, and carcinoma ex-pleomorphic adenoma.46
any submucosal spread. Moreover, the extent of the lesion into the deep tissues of the base of the tongue, vallecula, and glossopharyngeal sulcus is often only evident upon palpation. Assessment of the mandible range of motion and a thorough examination of the cranial nerves also must be performed, with an emphasis on sensation of the lower face and tongue, along with mobility of the tongue and palate. Cranial nerve deficits or the presence of trismus implies local invasion into the mandible or masticator spaces. All levels of the neck must be evaluated bilaterally, given the rich lymphatic network that drains the oropharynx and the high incidence of metastasis to the cervical lymph nodes.
Computed tomography (CT) and magnetic resonance imaging (MRI) are helpful in determining the extent of disease at the primary site or regionally. They are primarily indicated in the assessment of advanced-stage cancers where there is concern for involvement of the mandible, parapharyngeal space, prevertebral fascia, cervical nodes, or retropharyngeal nodes. CT scans are better able to evaluate bony structures and cervical lymphadenopathy, whereas MRI is better at evaluating soft tissue, such as the base of the tongue, parapharyngeal space, or prevertebral fascia.
Chest radiograph is useful as a screening for metastasis to the lungs, second primary cancers, and chronic changes associated with the use of tobacco. If a patient presents with significant lymphadenopathy that is in the inferior aspect of the neck (levels IV or VB) or bilateral in nature, a CT of the chest to detect lung metastases should be considered.
A panorex is a study complementary to the CT scan for the detection of mandibular involvement, and it is also critical in the assessment of the dentition prior to radiation therapy.
Positron emission tomography (PET) in conjunction with a contrast-enhanced CT of the neck can provide accurate anatomic details along with the biologic function about the primary tumor and regional disease. Because HPV-associated SCC frequently presents with smaller primary cancers and the nodal metastases are commonly cystic in nature, the additional functional characteristics provided by the 18F-FDG PET can improve the initial staging of these patients.3,49,50 Furthermore, although an increased frequency of distant metastases is not related to HPV status, HPV-associated OPSCC tends to disseminate to multiple organs that are not typically involved in non-HPV-associated metastatic HNSCC.51 These metastases also can manifest later in the course of the disease between 3 and 5 years after completion of treatment.51 Due to this atypical pattern of disease spread, initial and surveillance imaging needs to assess distant sites of disease. The benefit of PET/CT relative to a contrast-enhanced CT in the assessment of treatment response is still not clear. One of the challenges is the timing of the posttherapy PET/CT. The optimum timing after chemotherapy and radiation therapy is not known, but an interval of 12 weeks has generally been recommended to allow for a reduction in the radiation-induced inflammation and therefore improve the accuracy of the test.52
by HPV16 ISH but p16 positive by immunohistochemistry. About 45% of these cases can be attributed to the presence of some other (non-16) HPV type, as confirmed by wide spectrum ISH. The remaining discrepancies are probably due to false-positive staining of p16, which can be seen in basaloid carcinomas that are not related to HPV infection.62 To optimize HPV detection, a combination of p16 staining with ISH is the most effective. Because the sensitivity of p16 IHC approaches 100%, this is a reasonable first-line test to confidently eliminate those cases that are HPV negative. HPV16 ISH can then be run as a second-line assay for the p16-positive cases, and given a specificity of nearly 100%, the number of false positives from p16 staining alone will be reduced. The combination of these two assays identifies a subset of cancers that require a more rigorous analysis for non-HPV 16 oncogenic types. This third-line assay can either be consensus ISH probe set or PCR for the detection of transcriptionally active virus. This algorithm accurately detects HPV in the majority of cases, and although some tumors may require a more extended analysis, the overall expense is reduced because these cases are preselected.
Table 14.1 AJCC Staging for Squamous Cell Carcinoma of the Oropharynx | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree