28 Cancer of the Oropharynx
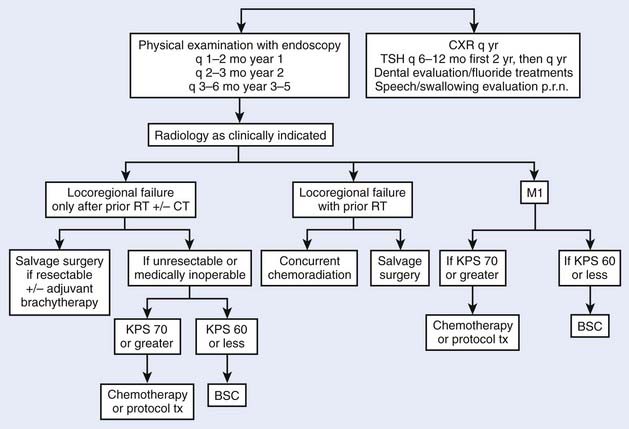
Tumors of the oropharynx comprise approximately 5000 new cases each year in the United States.1 The estimated incidence of pharyngeal cancers in the United States for 2007 was 11,800 new cases and 2180 deaths, with no breakdown by subsite of the pharynx.2 Patients with a history of tobacco or alcohol use are at increased risk for these tumors. Such a history can also be associated with other metachronous or synchronous tumors of the aerodigestive tract. The prognosis for oropharyngeal carcinoma depends on the location of the primary tumor and the stage at presentation. The most important cause of death is locoregional recurrence, which, if it occurs, usually manifests itself within 2 years. However, advances in radiation therapy as well as combined chemotherapy and radiotherapy have made local control much more successful, even for advanced primary site disease. Patients who survive are at risk for developing a second or third primary cancer in the upper aerodigestive tract or in the lower respiratory tract. The treatment strategies are numerous for these patients, and advances in organ preservation with attention to quality of life remain the major focus of research investigations.
Anatomy of the Oropharynx
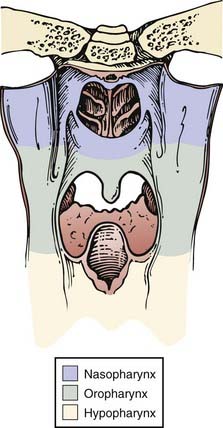
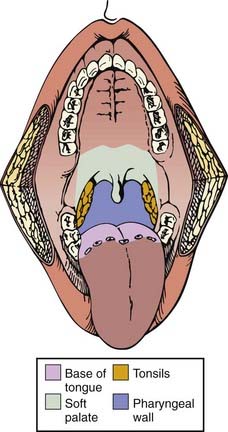
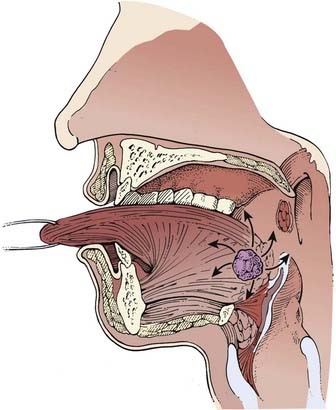
The pharynx is divided into the nasopharynx, oropharynx, and hypopharynx (Fig. 28-1). The oropharynx is located between the soft palate superiorly and the hyoid bone inferiorly. It is continuous with the oral cavity anteriorly and communicates with the nasopharynx above and the supraglottic larynx and hypopharynx below. Within the oropharynx are four different sites: soft palate, tonsillar region (fossa and pillars), base of tongue, and posterior and lateral oropharyngeal wall between the nasopharynx and the pharyngoepiglottic fold (Fig. 28-2).
Lymphatics of the Oropharynx
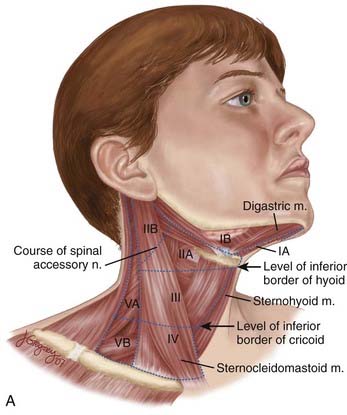
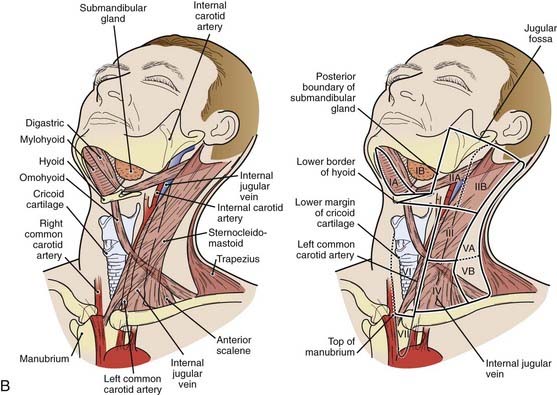
The lymphatic drainage of the neck was described in a classic paper by Rouviere3 in 1938 and has been refined by others.4,5 The lymph node groups are described by clinical levels I to V as depicted in Fig. 28-3.
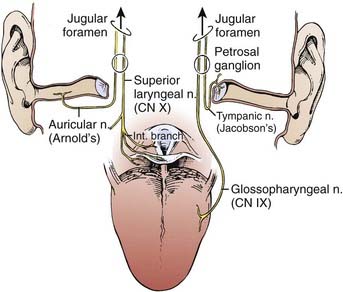
The primary drainage of the oropharynx is to the jugulodigastric (level II) node(s) located in the upper deep jugular chain. The tonsillar region, pharyngeal portion of the soft palate, lateral and posterior oropharyngeal walls, and base of tongue also are drained by the retropharyngeal and parapharyngeal nodes. These nodes are located in the retropharyngeal and parapharyngeal space that is closely related to cranial nerves IX through XII, the internal jugular vein, and the internal carotid artery at the base of skull. The retropharyngeal lymph nodes are subdivided into lateral (Rouviere) and medial nodal chains.6–8 The lateral nodes lie posterolateral to the nasopharynx and oropharynx, just medial to the internal carotid artery. The parapharyngeal lymph nodes are known also as the junctional nodes, owing to the junction of the spinal accessory (level V) and upper internal jugular lymphatic chains.
The probability of lymphatic metastasis is related to size and location of the primary tumor within the oropharynx. The order of progression of lymph node metastases usually proceeds superiorly, from the high cervical first-echelon nodes (level II) inferiorly to the midcervical and lower cervical nodes (levels III and IV). Skip metastasis can occur in which a particular lymph node level is bypassed, but this is very unusual. Candela et al.,9 from 1965 to 1986, evaluated 333 previously untreated patients with primary squamous cell carcinoma (SCC) of the oropharynx or hypopharynx to ascertain the prevalence of neck node metastases by neck level. The patients underwent classic radical neck dissections. Isolated skip metastases outside of level II, III, or IV occurred in only one patient (0.3%). Otherwise, level I or V involvement always was associated with nodal metastases at other levels.9 Metastases to the retropharyngeal nodes are most commonly associated with cancers of the nasopharynx and pharyngeal wall,10–12 but can also occur from other subsites, particularly with advanced disease.13,14 Notably, these metastases occur primarily along the lateral retropharyngeal nodal chains. Involvement of the medial chain is extremely rare.3–10
Tumors located in the midline (base of tongue, soft palate, and posterior pharyngeal wall) exhibit a higher propensity for bilateral lymphadenopathy. The probability of cervical node metastases, as demonstrated by clinical examination of the soft palate, tonsillar fossa, base of tongue, and oropharyngeal wall, is shown in Table 28-1.13
Pathology
More than 90% of tumors of the oropharynx are SCC, the remainder being malignant melanomas, minor salivary gland tumors, sarcomas, plasmacytomas, lymphomas, and other rare tumors.15 Benign and malignant tumors that can be found in the oropharynx are listed in Table 28-2. Metastases to the oropharynx do occur.16–19 Lymphoepithelioma is more common in the tonsillar region and base of tongue. The distinction between lymphoepithelioma and SCC is important, with the former likely to be particularly radiosensitive.20 Non-Hodgkin lymphoma is seen in approximately 5% of tonsillar malignancies and rarely is encountered in the base of tongue.
Table 28-2 Differential Diagnosis of an Oropharyngeal Mass
| Malignant |
| Benign |
Soft Palate
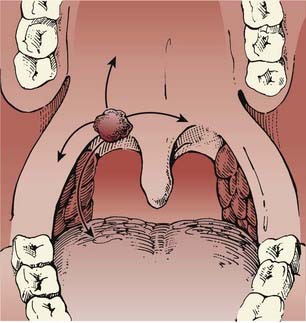
Tumors of the soft palate are almost exclusively found on the anterior surface (the oropharynx portion as opposed to the nasopharynx portion) (Fig. 28-4). In a study of 359 male US military veterans diagnosed with 424 cancers of the oral cavity and oropharynx, tobacco smoking was found to be more strongly associated with soft palate lesions than with lesions in more anterior sites.21 Tumors can extend to involve the tonsillar pillars and the base of tongue. Occasionally, these lesions may extend laterally and superiorly as far as the nasopharynx. Involvement of the palatine nerve can result in tumor tracking along this pathway, with extension into the cranium. Lymphatic involvement is primarily to level II. Lesions of the midline and uvula can result in bilateral nodal metastases more frequently than do lateralized lesions.
Tonsil
The most common location for a primary tumor of the oropharynx is the anterior tonsillar pillar and tonsil. Common presenting symptoms are ipsilateral referred otalgia, discomfort, poorly fitting dentures, or a sensation of a lump or foreign body in the throat. Lesions involving the anterior tonsillar pillar can appear as areas of dysplasia, inflammation, or a superficial spreading or exophytic lesion. Frequently, these lesions become endophytic, ulcerate, and can spread laterally to the buccal mucosa and directly to the retromolar trigone Fig. 28-5. Inferior extension to the base of tongue is common. Perez et al.22 reported that of 218 patients presenting with SCC of the tonsillar region, the soft palate was involved in 131 (60%) and extension to the base of tongue occurred in 120 (55%). Superior extension can involve the soft and hard palate. Medial extension can involve the oral tongue. The close proximity of the anterior tonsillar pillar to the mandible places the periosteum and bone at risk in advanced cases. Posterior extension with destruction of the tonsillar pillars can involve the pterygoid muscles, with subsequent trismus and pain. The lymphatic drainage is primarily to level II but can involve level I and the retropharyngeal and parapharyngeal nodes.
The probability of clinical lymph node involvement is greater with tumors of the tonsillar fossa, especially contralateral involvement, in contrast to that of the tonsillar pillar. The lymphatic drainage depends on the location of the primary tumor. Lindberg13 describes nodal metastases in 76% of patients with tonsillar fossa tumors. The most common nodal group was level II. Contralateral lymph nodes were detected in 11% of patients. By contrast, tumors of the anterior tonsillar pillar or retromolar trigone region have an incidence of ipsilateral lymph node metastases of 45%, level II being the most common node-bearing region. Contralateral adenopathy was present in only 5% of patients.
Base of Tongue
SCC of the base of tongue is highly insidious. The base of tongue is almost devoid of pain fibers, and frequently these tumors are asymptomatic until they have progressed significantly. Many who present with a neck node are found, on examination, to have a base-of-tongue lesion. Visualization of this area on physical examination is difficult, and so a lesion often is missed.23 Patients may experience the sensation of a mass or discomfort in the throat, with bleeding and pain at later stages. Patients also might experience difficulty in swallowing or with speech. Occasionally, referred otalgia is the first symptom. If the size of the primary tumor increases such that it involves the pterygoid muscles, trismus can result.
Extension anteriorly can involve the oral tongue, superior and lateral extension can involve the tonsil, and inferior extension can involve the vallecula, epiglottis, and pre-epiglottic space (Fig. 28-6). Locally advanced base-of-tongue tumors can infiltrate the deep muscle and cause fixation.
Diagnostic Evaluation
History
The history should be part of a comprehensive evaluation of any patient with head and neck cancer. Patients usually present with pain and dysphagia and, occasionally, referred otalgia. If the history strongly reveals tobacco and alcohol use, efforts should be made to determine whether the patient is an alcoholic and continues to smoke. Alcoholic smokers are at risk for other chronic diseases of the heart, lung, peripheral vascular system, and liver, and may present with signs of malnutrition. Before the institution of any therapeutic modality, patients should cease alcohol and tobacco use. It may be necessary to guide the patient toward cessation programs. Clearly, those who discontinue smoking will better tolerate treatment and obtain a better result.24
Physical Examination
Specific aspects of the physical examination should include evaluation of the lesion (exophytic or infiltrative), tongue mobility, and palatal motion. Fixation of the tongue will result in incomplete protrusion or deviation of the tongue to the side of tumor involvement. In patients with a smoking and drinking history, three sites have a greater propensity for developing carcinoma than do others in the oral cavity and oropharynx: floor of mouth, ventrolateral tongue, and lingual aspect of the retromolar trigone and the anterior tonsillar pillar.25,26
For tumors of the tonsil or lateral pharyngeal wall, the examiner should test for anesthesia in the distribution of the ipsilateral mental nerve (V3). Any abnormality might suggest involvement of the inferior alveolar nerve in its pathway as it courses through the mandible or the base of skull and may direct the appropriate imaging study.20 The tonsil is adjacent to the ascending ramus of the mandible, and posterolateral to the tonsil is the parapharyngeal space. Tumors may extend into this area and be palpable in the neck on bimanual palpation. Owing to the relationship of the anterior tonsil (palatoglossus muscle) and the base of tongue, tonsillar cancers frequently extend into this region.
Initial Work-Up and Radiographic Evaluation
In addition to a history and physical examination, a complete blood cell count, screening profile, and chest roentgenogram are recommended (Fig. 28-8). Biopsy of a suspicious lesion is necessary to confirm the diagnosis.27 A more detailed metastatic work-up is indicated only when strong clinical or laboratory suspicion of metastatic disease exists.
Staging
The current staging criteria for tumors of the oropharynx, as defined by the American Joint Committee on Cancer (AJCC),28 are listed in Table 28-3. This is a clinical staging system and not a pathologic staging system. If radiographic information reveals a discrepancy from clinical staging, this should be noted. Current staging allows the radiographic findings to factor into the clinical staging designation.
Table 28-3 2002 AJCC Classification of Oropharyngeal Cancer

Early-Stage Disease: Site-Specific Management Strategies, Results, and Outcomes
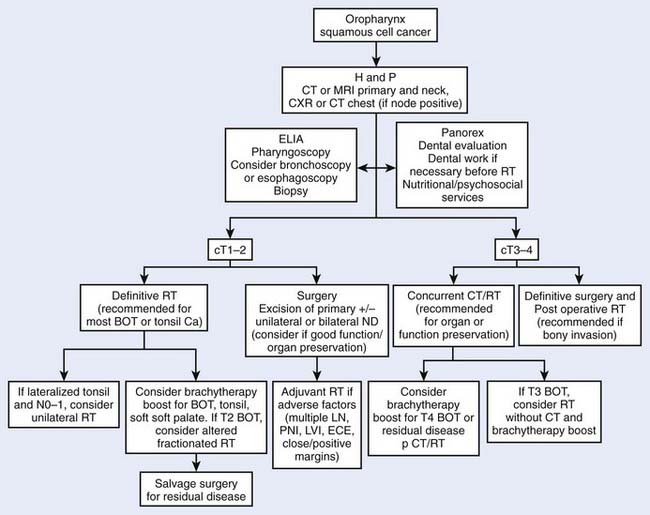
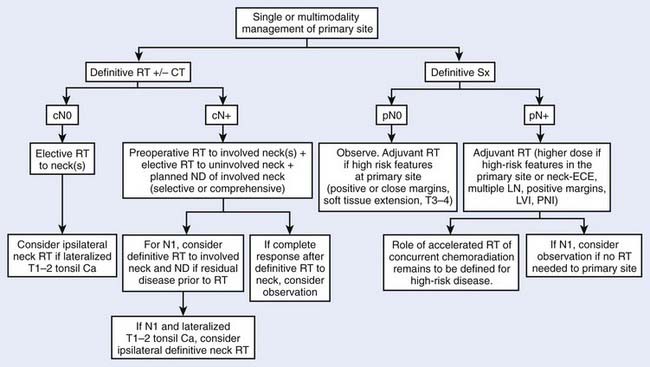
Treatment decisions depend both on the ability of a particular modality to control the primary tumor and on the state of the neck and its associated morbidity. In general, early stage disease can be treated by either radiation therapy (RT) or surgery, whereas more advanced lesions often are treated by combinations of these methods. RT is chosen more often than surgery for most early lesions because the cure rates are high and the functional outcome is better. Chemotherapy generally is reserved for patients with advanced disease or for certain organ-sparing protocols. In the following sections, the management guidelines, outcomes, and results of oropharyngeal tumors will be reported by subsite of the oropharynx (Figs. 28-9 and 28-10). Because the management of advanced disease is, in many cases, similar for the subsites of the oropharynx, they will be discussed together in the next section.
Soft Palate
Selection of Therapeutic Modality
For early stage lesions of the soft palate, either surgical resection or RT has provided excellent local control. Most patients will be treated with RT because the results are excellent and the probability of a good functional result is better. Also, because many lesions are near the midline, radiation treatment of the primary site and both sides of the neck is easy (see Radiation Therapy Techniques). In general, more morbidity ensues with surgical therapy to these same areas, especially if postoperative radiation becomes necessary.
Outcomes and Results
Definitive External Beam Radiotherapy
Two major series examine the use of EBRT alone or in conjunction with planned neck dissection. Erkal et al.29 analyzed 107 patients with SCC of the soft palate treated at the University of Florida between 1965 and 1996. Ninety-nine patients received external beam irradiation alone, while 8 patients (7%) received external beam RT followed by an interstitial brachytherapy boost. Patients received a median dose of 62.4 Gy via once-daily fractionation or 76.8 Gy via hyperfractionated techniques. The 5-year local control rates for T1 through T4 tumors were 86%, 91%, 67% and 36%, respectively. Ultimate 5-year local-regional control (LRC) after surgical salvage for Stages I through IV was 90%, 92%, 84%, and 60%, respectively. On multivariate analysis, overall treatment time significantly affected local control (P = .0002), regional control (P = .01) and overall survival (P = .04). Overall, 3% of patients treated with RT and 11% (3/27) patients treated with RT followed by neck dissection sustained severe late complications. Weber et al.30 reviewed the experience at the University of Texas M.D. Anderson Cancer Center. Treatment to the primary site consisted of RT for 150 patients, surgery alone for 28 patients, and combined therapy for 10 patients. Local control rates for T1 through T4 lesions were 91%, 77%, 77%, and 35%, respectively. Local control was obtained in 88% of patients with N0 necks and in 77% of patients with nodal involvement. In addition, these investigators found that patients with tumor extension to the tongue base, midline tumors, or tumors that extended across the palatine arch had inferior survival compared with those who did not exhibit those features (P < .05).30 Similar results have been reported in other single-institution retrospective series.31
Combined External Beam Radiation Therapy and Brachytherapy Implant
The use of a brachytherapy implant for small soft palate lesions has been reported extensively by French investigators. Esche et al.32 describe 43 patients with carcinoma of the soft palate and uvula who were treated by interstitial implant and EBRT. Patients received 5000 cGy external irradiation to the oropharynx and neck, followed by 2000 to 3500 cGy delivered by a low-dose rate (40 to 100 cGy/hour) interstitial iridium-192 (192Ir) implant. This therapy yielded a local control of 92%, with no local failures in 34 T1 primary tumors. One serious complication was seen. Overall actuarial survival was 60% at 3 years and 37% at 5 years, but cause-specific survivals were 81% and 64%, respectively. The leading cause of death was other aerodigestive cancers, with an actuarial rate of occurrence of 10% per year after treatment of a soft palate cancer.
Mazeron et al.33 reported on a subset of patients with early stage tumors who received EBRT to the primary tumor and neck nodes to a dose of 4500 cGy, followed by 3000 cGy delivered by a 192Ir implant to the primary tumor. Local control was 85% for soft palate tumors. Regional control was 97% for patients with N0 disease and 88% for patients with N1 through N3 disease. Soft tissue ulceration occurred in 17 patients, all of whom healed spontaneously.
Pernot et al.34 reported on 277 patients with velotonsillar cancer (oropharyngeal cancer excluding base of tongue and valleculae) who were treated by brachytherapy either alone (14 patients) or combined with external beam irradiation (263 patients) using an after-loading 192Ir technique. Thirty-five percent of the patients had soft palate lesions. Of the patients treated for early lesions in the soft palate, the local control rates for T1 to T2N0 and T1 to T2N1 to N3 were 90% and 86%, respectively. No local recurrence was detected after 3 years.
External Beam Radiation Therapy versus External Beam Radiation Therapy Plus Brachytherapy
The need for an implant was analyzed retrospectively by Mazeron et al.35 who reported T1 and T2 carcinomas of the soft palate and uvula treated definitively by EBRT alone (16 patients), 192Ir implant alone (14 patients), or a combination of the two methods (29 patients). Two techniques of implantation were used: the guide-gutter technique (33 patients) and the plastic-tube technique (10 patients). Local failure was 25% after EBRT alone, 0% after 192Ir implant alone, and 18% after combined therapy. No local failures were seen with the plastic tube technique compared with 15% for guide gutters. Only two nodal failures were observed (3%). Crude 5-year disease-free survival (DFS) was 33%. Severe complications were limited to one incident of osteonecrosis, one soft-tissue necrosis, and one case of partial palatal incompetence. Xerostomia was reduced when implantation was used for part or all of the treatment. This is not conclusive proof that an implant is required, and these results may be difficult to duplicate without the requisite expertise in brachytherapy. Clearly, patient selection factors are important in these results.
Fractionated High Dose Rate or Pulsed Dose Rate Brachytherapy
In an effort to minimize occupational radiation exposure and decrease the patient isolation period while minimizing normal tissue complications, Levendag et al. reported an initial experience combining EBRT with high dose rate (HDR—100 cGy/minute) or pulsed dose rate (PDR) brachytherapy in 38 patients—19 soft palate tumors (14 T1 to 2) and 19 tonsillar cancers.36 Twice-a-day fractions of 3.0 to 5.4 Gy HDR to a dose of 15 to 27 Gy or 4 × 2 Gy to 8 × 1 Gy per day of PDR to a dose of 20 to 28 Gy was delivered within 1 to 2 weeks after completion of EBRT (46 to 50 Gy, median cumulative dose of 66 Gy). At a mean follow-up of 2.6 years, 87% of soft palate tumors were locally controlled. The incidence of grade 3 mucositis or ulceration was no different between patients treated with PDR versus HDR and similar to those reported after low dose rate brachytherapy.
Tonsillar Region
Selection of Therapeutic Modality
Radiation Therapy
The use of RT as the definitive treatment for tumors of the tonsillar fossa is appropriate for T1, T2, and T3 (exophytic) tumors. For infiltrative or endophytic T3 or T4 lesions, either an organ-preserving approach involving concurrent chemotherapy or, in selected instances, surgery combined with postoperative RT (or concurrent chemoradiation) should be used. The target volume of the ipsilateral treatment should include the primary lesion with at least 2-cm margins, the ipsilateral jugular vein, and lateral retropharyngeal lymph nodes. If disease extends to the base of tongue, then EBRT alone is not as effective as EBRT plus implant to the tongue.37 In this case, full-dose EBRT is delivered, followed by an interstitial 192Ir implant as a boost to the tongue portion of the target.
For early-stage, ipsilateral lesions with N0 or N1 nodal involvement, the primary site and ipsilateral neck can be treated while sparing the contralateral side, thus, optimizing the patient’s oncologic treatment and treatment-related morbidity. In the modern era, this is achieved with either three-dimensional conformal radiation therapy (3DCRT) or an intensity-modulated radiation therapy (IMRT) plan (see Radiation Therapy Techniques). In general, most T1 to T2 lesions in patients with an N0 or N1 neck can be treated with ipsilateral fields. Lesions that cross the midline, involve the tongue base, or involve N2 or more advanced neck disease should be treated to both the ipsilateral and contralateral neck. Treatment planning must ensure protection of critical normal structures including the spinal cord and larynx. A computed tomographic (CT) scan is performed for treatment planning.
Outcomes and Results
Surgery Alone
The use of surgery as the sole treatment for early tonsillar disease is not reported frequently. However, excellent local control rates ranging from 80% to 90% have been reported.34,38,39 When there is extension to the lateral pharyngeal wall or base of tongue, local recurrence approaches 33% and 47%, respectively.40–42 The degree to which local control can be obtained depends on the extension of disease outside the tonsillar fossa.
External Beam Radiation Therapy Alone
No definitive randomized studies comparing surgery versus EBRT have been reported. However, no obvious differences between these modalities in LRC or absolute survival can be determined based on the reported literature.38,43–54 EBRT alone has become the treatment of choice for most early stage lesions. If local control is maintained in patients by EBRT alone, the greatest risk to these patients is the development of future aerodigestive malignancies.55 In general, the results using conventionally fractionated EBRT alone (1.8 to 2.0 Gy/dose during 6.5 to 7 weeks to doses of 65 to 70 Gy) are excellent for early stage tumors.39,47–49,56
Mendenhall updated the University of Florida experience using definitive radiation to the primary site for treatment of 503 tonsillar cancers during a 38-year period, with a minimum 2-year follow-up.57 Patients were treated with continuous course radiation (n = 177, median total dose 66 Gy) or using hyperfractionation (n = 326, median total dose 76.8 Gy). Eighteen patients received induction chemotherapy, 39 received concomitant chemotherapy, 198 underwent planned neck dissection, and 106 underwent an interstitial brachytherapy boost. Five-year rates of local control were as follows: T1 (n = 75) 88%; T2 (n = 191) 84%; T3 (n = 151) 78%; and T4 (n = 86) 61%. Of the 95 local recurrences, 26 patients (27%) were successfully salvaged. The ultimate local control rates at 5 years were T1 94%, T2 91%, T3 82%, and T4 68%. Five-year cause-specific survivals by 1998 AJCC stage (5th ed.) were as follows: I 100%, II 86%, III 84%, IVA 73%, and IVB 46%. Absolute overall survival (OS) at 5 years was as follows: I 54%, II 61%, III 62%, IVA 57%, and IVB 33%. Only three patients required hospitalization during or shortly after RT; however, 9% (46/503) developed severe late complications including osteoradionecrosis requiring mandibulectomy (n = 15), dysphagia requiring gastrostomy (n = 18), bone exposure necessitating debridement and hyperbaric treatment (n = 10), orocutaneous fistula (n = 2), fatal radiation-induced sarcoma (n = 1) and fatal aspiration pneumonia (n = l).
Remmler et al.40 reported the results in 160 patients in whom EBRT was the sole therapy for the majority of patients. Primary tumor control rates were 100% for T1 lesions, 89% for T2, 68% for T3, and 24% for T4. In addition, control of cervical metastases by RT was excellent (95%). When a planned neck dissection was performed 5 weeks after RT, the control of cancer in the neck was 100%. The incidence of distant metastases was 10% and was not affected by the selection of therapy. The 2- and 5-year determinate survival figures for 112 patients treated with RT alone were 67% and 48%, respectively, whereas 31 patients treated with RT followed by neck dissection achieved a 5-year survival rate of 48% (Table 28-4).
Table 28-4 Tonsillar Carcinoma: Local Control and Survival with EBRT, With or Without Brachytherapy Implant
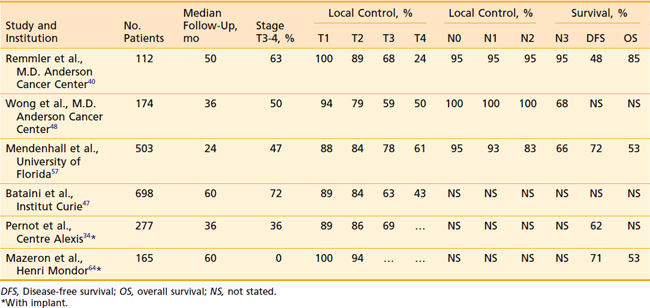
Lower doses or split-course radiation results in poorer LRC.58 Using RT alone, Bataini et al.47 reported that in tumors arising from the glossopalatine sulcus, characterized by involvement of the tongue, inferior local control is achieved compared with similar therapy for tumors arising from other sites within the tonsillar region.
Ipsilateral External Beam Radiation Therapy
For patients with T1 and T2 lesions, treatment of the ipsilateral neck alone usually is possible without having to irradiate the contralateral neck, which minimizes irradiation to the contralateral salivary gland and reduces the incidence of xerostomia. Eisbruch has demonstrated that patients treated unilaterally report less xerostomia and better quality of life compared with those treated with intensity-modulated RT delivery of bilateral radiation with contralateral parotid sparing.59 However, careful patient selection is required to minimize the risk for contralateral neck failure. Two recent major series document excellent outcomes using ipsilateral neck EBRT.
O’Sullivan et al. reported the results of 228 tonsillar carcinomas treated with ipsilateral RT during a 20-year period at Princess Margaret Hospital.60 Eligible patients typically had T1 or T2 tumors (191 T1-2, 30 T3, 7 T4) with N0 (133 N0, 35 N1, 27 N2 to 3) disease. During this period, only 16 patients were treated surgically. Radiation was typically delivered with wedged-pair cobalt beams and ipsilateral low anterior neck field delivering 50 Gy in 4 weeks (90% isodose line) to the primary volume. At a median follow-up of 5.7 years, the 3-year local control rate was 77%, regional control was 80%, and cause-specific survival was 76%. Contralateral neck failure occurred in 3% (8/228). All patients with T1 lesions or N0 neck status had 100% contralateral neck control. Patients with a 10% or greater risk for contralateral neck failure included those with T3 lesions, lesions involving the medial one third of the soft hemipalate, tumors invading the middle third of the ipsilateral base of tongue, and patients with N1 disease.
Jackson et al. reported an 18-year experience of 178 patients receiving ipsilateral treatment using limited fields for tonsillar cancers.61 Patients presented primarily with T1 to T2 (117/178 [66%]) and N0 (101/178 [57%]) disease, but 29% (52/178) had T3 tumors and 30% had N1 disease, with 63% presenting with stage III to IV disease. Sixty Gy per 25 fractions (50 to 66 Gy) was delivered to gross tumor volume with a 1-cm margin and first echelon lymph nodes using two or three wedged fields. The length of follow-up was not stated. The rates of LRC and contralateral neck recurrence by stage were as follows after ipsilateral RT: I (n = 23), 91%, 0%; II (n = 43), 74%, 2%; III (n = 82), 51%, 4%; IV (n = 30), 53%, 0%. Patients with N0 (n = 101) or N1 (n = 54) disease had contralateral failure rates of 0% and 4%, respectively. None of the 23 patients with N2 to N3 disease had contralateral failure; however, the determinate risk for contralateral failure may have been obscured by the 52% incidence of ipsilateral failure. The authors were unable to relate the risk for contralateral neck failure according to the degree of tumor extension along the glossopalatine fold due to the retrospective nature of the study. The overall rate of local control was 75% (T1-2, 84% and T3-4, 58%) and OS was 56%.
External Beam Radiation Therapy and Brachytherapy
Pernot et al.34 reported on 277 patients, 101 of whom had advanced disease (T3). The 5-year local control, disease-specific survival, and OS rates by T stage (T1, T2, T3) were as follows: local control, 89%, 86%, and 69%, respectively; disease-specific survival, 78%, 62%, and 46%, respectively; and OS, 62%, 53%, and 43%, respectively. No local recurrence was detected after 3 years. In a later update of 361 cases, the 5-year outcomes of patients with tonsil, posterior pillar, or soft palate cancers (group A) were compared with those involving the anterior pillar and glossopharyngeal sulcus (group B).46 Local control was better in group A patients compared with those in group B as follows: T1 94% versus 75%; T2 93% versus 67%; and T3 71% versus 51%, respectively. Disease-specific survival and OS was also better in group A as follows: T1: 88%, 65% versus 55%, 48%; T2 78%, 63% versus 43%, 38%; and T3 53%, 49% versus 27%, 27%, respectively. Multivariate analysis revealed that a treatment interval of less than 20 days between EBRT and brachytherapy and an overall treatment time of less than 55 days yielded better outcomes. Complications in this patient population appeared to be related to a total dose greater than 80 Gy, dose rate greater than 70 cGy per hour, treated surface area greater than 12 cm2, treatment volume greater than 30 cc, and absence of leaded protection.62
Levendag et al. reported on 248 patients with Stage T1-T3, N0-N+ SCC of the tonsillar fossa and/or soft palate treated at Erasmus Medical Center between 1986 and 2001.63 One hundred four patients were treated with external RT (median dose 46 Gy) followed by a brachytherapy implant, primarily using high-dose-rate (15 to 30 Gy at two daily fractions of 3 to 4 Gy to 15 to 30 Gy, n = 31) or pulsed-dose-rate (eight daily fractions of 1 to 2 Gy to 18 to 30 Gy, n = 62) treatment schedules. Patients who were node-positive underwent unilateral or bilateral neck dissection at the time of brachytherapy implant. Eighty-six patients who were felt to be unsuitable for brachytherapy at presentation (e.g., parapharyngeal extension) underwent surgery of the primary tumor and neck dissection, followed by postoperative RT to 50 to 70 Gy, depending on pathologic risk factors. Local control rates at 5 years for the definitive RT and surgical cohorts were 88% and 88%, respectively. Regional control, DFS, and OS rates were 93% versus 85%, 57% versus 52%, and 67% versus 57%, respectively. Significant late side effects in the brachytherapy group versus the surgery group included and trismus (1% versus 21%, P = .005) ulceration (39% versus 7%, P = .001). Ulceration healed spontaneously in 88% after a mean period of 6 months.
External Beam Radiation Therapy versus External Beam Radiation Therapy Plus Brachytherapy
Mazeron et al.64 reported on 165 T1 to T2 SCCs of the faucial arch. Because of institutional policy changes, these authors were able to compare patients who received EBRT alone with those with EBRT and implantation. Those who received an implant were first treated by EBRT to the tumor site and neck areas (4500 cGy in 25 fractions during 5 weeks) and then received a 3000-cGy low-dose-rate 192Ir implant. For patients with clinically positive nodes, either additional 2500- to 3000-cGy electron beam irradiation to the nodes or neck dissection was added. Both local control (77% versus 94% at 5 years; P < .01) and DFS (56% versus 71%; P = .03) were improved for the implant group. No randomized study has shown whether this combined approach is superior to EBRT alone. Nonetheless, even advocates of the use of EBRT alone agree that the addition of an implant can improve local control when disease extends into the tongue.37
High Dose Rate Brachytherapy
High dose rate brachytherapy as boost treatment with external beam radiation has been reported in two small series to offer excellent local control for early stage tonsil, base of tongue, and soft palate tumors with local control of 83% to 87% but lower with T3 to T4 tumors, 42% to 47%.36,65 Fractionated high dose rate brachytherapy using b.i.d. treatments of 1.2 to 3.0 Gy fractions was combined with EBRT to total cumulative doses of 66 to 72 Gy. Successful surgical salvage was 50% to 60% among those who failed locally with no obvious increase in surgical complications such as fistula or flap necrosis. Rates of serious complications (primarily soft tissue ulcer and mandibular osteoradionecrosis) were reported in 10% to 16% of patients and were similar to those reported by low dose rate brachytherapy reports.
Base of Tongue
Selection of Therapeutic Modality
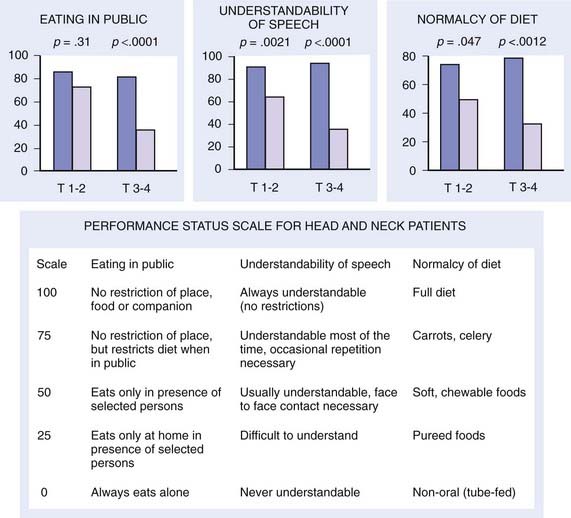
Early stage base-of-tongue disease is successfully treated with either definitive RT or surgery. The results are equivalent for local control and survival. The morbidity of a surgical procedure must be weighed against the morbidity of RT. In the overwhelming majority of cases, RT is selected because it provides a better functional result and quality-of-life (QOL) outcome (Fig. 28-11).
Radiation Therapy
Our preferred strategy entails EBRT followed by an interstitial 192Ir implant as a boost to the base of tongue. We believe that this offers the most consistent excellent local control and best function results compared with either EBRT alone or to primary surgery. External beam radiation is delivered to a dose of 5400 cGy in 30 fractions (180 cGy/fraction). A brachytherapy boost to the tongue base (2000 to 3000 cGy) is performed approximately 3 weeks after EBRT (5000 to 5400 cGy). The implant consists of 192Ir after-loading catheters placed with a looping technique,66,67 which involves the percutaneous introduction of curved trocars by means of a submental approach through the base of tongue. The catheter is threaded through the trocar and looped back through an adjacent trocar, creating a loop in the tongue. For patients with disease extension toward the pharyngoepiglottic fold, lateral loops are added to encompass this region.66 Both ends exit the skin of the neck. An array of loops is fashioned to encompass the target volume plus a 1.0- to 1.5-cm margin. The spacing between each end of the loop is 1 cm and between each plane is 1.0 to 1.5 cm (see Radiation Therapy Techniques). As a safety precaution, a temporary tracheostomy is performed in patients immediately before the implantation. A temporary nasogastric feeding tube is placed at the completion of the procedure. The neck is managed by RT alone in the patient with N0 or N1 disease. For patients with more advanced neck disease, chemotherapy is given concomitantly with RT. When a neck dissection is planned, the implantation and dissection are performed during the same anesthetic period. Patients are loaded several days after the procedure, after the majority of swelling has subsided and patients are able to suction themselves and perform tracheostomy care. Efforts are made to protect the palate by use of a customized shield that is under patient control.
Surgical Therapy
By contrast, patients with nodal metastases routinely will undergo a surgical procedure in the neck if the primary tumor is being treated by radiation alone. The neck dissection preferably is performed at the completion of RT. If the primary tumor is being managed surgically, surgery followed by RT is preferred. In our practice, patients generally undergo a combination of EBRT and a brachytherapy implant (discussed later in this chapter). In this setting, the implant and the neck dissection are carried out during the same anesthetic period, approximately 3 weeks after the EBRT is completed. Andersen et al.68 have shown that preservation of the spinal accessory nerve in a modified neck dissection in patients with clinically evident nodal metastases was not associated with increased risk for treatment failure in the dissected neck.
Base-of-tongue tumors may be resected transorally or through a mandibulotomy and transhyoid pharyngotomy. The transoral approach is indicated only for small, well-circumscribed lesions that are located superficially. The mandibulotomy technique allows superior access and frequently is combined with a graft.69 When a patient has evidence of bone invasion or close encroachment of tumor to bone, frequently a mandibulectomy is performed. The use of a flap or plate with this procedure depends on the age of the patient, the amount of tissue resected, and the anticipated functional or cosmetic outcome.
After surgery, patients will spend a great deal of time learning to swallow and avoiding aspiration. If the dysfunction is significant, the patient may require placement of a percutaneous endoscopic gastrostomy (PEG), which can be performed at the time of surgery.70 A prosthesis may have to be constructed to aid speech and swallowing. Speech rehabilitation is started as soon as possible while the patient is still in the hospital.
Postoperative Adjuvant Radiation Therapy
After careful examination of the resected specimen by the pathologist, attention should be paid to the size of the primary tumor, histology, surgical margins, evidence of perineural extension, and lymph nodes. Patients with small tumors (stage T2), clear negative margins, no evidence of perineural extension, and histologically negative lymph nodes do not require postoperative RT. RT generally is used when the surgical wound is seeded, when close or positive margins are present, and in the presence of extranodal tumor spread (extracapsular extension), multiple positive nodes, or extensive vascular or perineural invasion.71 Because larger primary tumors are poorly controlled locally with surgery alone, postoperative RT is suggested in this group of patients. The retropharyngeal lymph nodes are included in the treatment of the primary tumor.72 Owing to the high probability for bilateral neck disease, RT is used electively to control the area of unoperated contralateral neck.
The interval between surgery and RT was examined by Vikram,73 who reports that a delay of 7 weeks or more was associated with increased locoregional failure and decreased survival. The neck disease recurrence rate was 2% if the postoperative RT was started within 6 weeks compared with 22% at later than 6 weeks. A reanalysis by Schiff et al.74 suggests that a prolonged delay in postoperative RT does not have a negative impact on LRC as long as appropriate tumoricidal doses of more than 6000 cGy are employed. Seventy-three percent in the delayed treatment group received doses less than 56 Gy, accounting for their increased risk for locoregional failure. Bastit et al. reported no difference in LRC or survival in a retrospective study of 420 oropharyngeal and hypopharyngeal carcinomas who were treated with similar doses (59 Gy/6 weeks) either within (n = 219) or after 30 days (n = 201) from surgery.75 However, Ang et al. reported that among high-risk patients (positive margins, extracapsular nodal extension, or multiple lymph nodes), a duration of less than 11 weeks from the end of surgery to the end of radiation resulted in better LRC and survival compared with those treated during a longer period.76 We recommend starting radiation as soon as possible.74
Outcomes and Results
Surgery Alone versus Radiotherapy
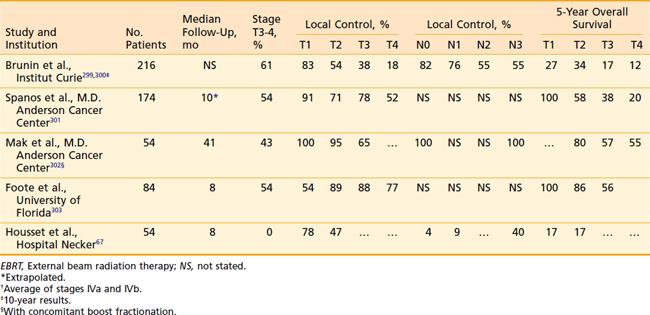
Surgical results for early base-of-tongue tumors reflect relatively high local control rates—from 75% to 85%.75,76 Primary EBRT, alone or followed by a planned neck dissection, also has a high local control rate of approximately 80% to 90% for T1 to T2 lesions and 70% to 85% for T3 tumors (Table 28-5). Survival for RT is similar to that after surgery, but with a lower risk for severe complications than surgery.77
Data from numerous authors have shown that the local control for early stage tumors is in the range of 80% to 100% when treated with combined EBRT and brachytherapy implant (Table 28-6). Pernot et al. reported a 5-year respective local control of 93% and 72% for T1 (n = 14) and T2 (n = 27) base-of-tongue tumors treated with a combined EBRT and brachytherapy approach. Corresponding 5-year disease-specific survivals were 76% and 62%, respectively.46
Table 28-6 Base-of-Tongue Carcinoma: Local Control and Survival With EBRT and Brachytherapy Implant Boosts
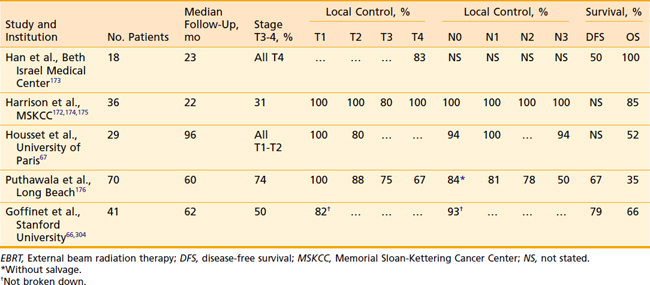
Housset et al.67 have evaluated a comparison among surgery plus postoperative RT, EBRT plus implantation, and EBRT alone for a series of patients with T1 to T2 base-of-tongue lesions. This series is unique in that it attempts to address the issue of which management strategy is optimal. Demographic and oncologic characteristics of the patients were well balanced except that, among the EBRT-alone group, there were significantly more patients with exophytic (more favorable) lesions. Despite this imbalance, the patients who received EBRT alone had approximately twice the local failure rate as those in the other two groups (40% versus 20%). This study suggests that EBRT plus brachytherapy is the preferred technique.
Pharyngeal Wall
Outcome and Results
Surgery Alone
The use of surgical resection alone for pharyngeal wall tumors has been reported by Guillamondegui et al.78 Twenty-eight percent of patients had recurrent tumors above the clavicles. Salvage in the form of RT or surgery was successful in less than one third of those patients. Patients with positive retropharyngeal nodes had an increased rate of distant metastases (22%) compared with those who did not have positive nodes (15%).
Definitive Radiation Therapy Alone
Hull et al.79 from the University of Florida retrospectively reviewed their experience with definitive radiation for pharyngeal wall tumors treated between 1964 and 2000. Thirty-seven percent of patients had oropharyngeal wall tumors, while the remainder was of hypopharyngeal origin. Sixty-four percent of patients were treated with a hyperfractionated technique, and 11 patients also received chemotherapy. Five-year local control rates for T1, T2, T3, and T4 tumors were 93%, 82%, 59%, and 50%, respectively. On multivariate analysis, factors associated with improved LRC included twice-daily fractionation (P = .0009), Stage I to II disease (P = .0051), and oropharyngeal primary site (P = .0193). The same group previously reported the effect of the use of once-daily to twice-daily fractionation on local control in 99 patients with carcinomas of the hypopharyngeal or oropharyngeal wall or both. The local control rates for patients treated with once-daily versus twice-daily fractionation were, for T1 lesions, 100% versus 100%; for T2 lesions, 67% versus 92%; for T3 lesions, 43% versus 80%; and for T4 lesions, 17% versus 50%. These investigators also examined their former technique (posterior border placed at middle of the vertebral body when the portals were reduced off the spinal cord) compared with their current, modified technique (posterior border placed at posterior edge of the vertebral body). The local control rates were 100% each for T1 lesions; 57% versus 100% for T2 lesions; 46% versus 73% for T3 lesions; and 29% versus 75% for T4 lesions.80
Meoz-Mendez et al.81 reported on 164 patients treated at the M.D. Anderson Cancer Center for carcinomas of the hypopharynx and pharyngeal walls. Local control rates for T1 through T4 lesions were 71%, 73%, 61%, and 37%, respectively. For patients with T3 and T4 tumors, the combination of surgery and RT was superior to RT alone (75% local control versus 51%).
Surgery and Radiation Therapy
Marks et al.82 retrospectively compared the results of treatment in 51 patients after low-dose (2500 to 3000 cGy) preoperative RT and surgery to the results in those who had definitive RT. No difference was found in local control or survival (17%); however, the surgery plus RT group experienced a significant number of complications (fistula, 31%; carotid rupture, 14%; operative mortality, 14%). The same authors updated their experience with a group consisting of 89 patients.83,84 These patients were treated with high doses of radiation (5000 to 7200 cGy) either for preoperative intent or for definitive therapy. Treatment outcome, survival, and tumor and nodal control were better for the RT-plus-surgery group than for the RT-alone group. The patterns of relapse differed for the two groups, with low-dose preoperative irradiation and surgery offering greater control of the primary tumor and high-dose irradiation achieving better control of nodal disease. To draw any conclusions from this article is difficult, as the reported study spanned almost 20 years, during which time many technologic advances were made in radiation oncology, anesthesia, and surgery.
Spiro et al.85 reviewed a 12-year experience with 295 patients treated for SCC of the pharynx at the Memorial Sloan-Kettering Cancer Center. Of these patients, 78 patients had lesions in the posterior wall. Surgery was the definitive therapy for the primary tumor in 73%. A second group of 21 patients with more extensive tumors required a laryngectomy and complex reconstruction, often with postoperative RT, and five had lesions that were implanted after access was provided by a mandibulotomy. The cumulative 5-year survival for the entire group was 32% and ranged from 44% in those with favorable lesions to 15% in those with extensive tumors. The overall complication rate was 50%. For the group that received an implant, local control was excellent.
Julieron et al.86 from the Institut Gustav-Roussy reported their results among 77 patients treated surgically between 1984 and 1995. Twenty-three of these patients had been previously irradiated, and all patients who were previously untreated underwent postoperative radiation. Of the 54 patients who were previously untreated, 89% were locally controlled and 78% were locoregionally controlled, with a 5-year survival rate of 35%.
The use of a brachytherapy implant (either 192Ir or iodine-125 [125I]) and EBRT was reported by Son and Kacinski87 for a small group of patients. The local control rate was 86% and actuarial survival was 82% at 5 years. These results are impressive but must be confirmed. In general, results in patients with extensive lesions still leave much to be desired, despite radical surgery and aggressive RT, and creative brachytherapy techniques warrant further investigation.
Advanced Disease: Management Strategies, Results, and Outcomes
Altered Fractionated Radiation
Withers et al. analyzed outcomes from nine centers using widely different dose-fractionation radiation regimens treating a total of 676 tonsil carcinomas (range of total dose 50 to 72 Gy, dose per fraction 1.8 to 3.3 Gy, and overall treatment time 3 to 8 weeks).88 Total dose and treatment duration were significant treatment parameters that impacted on local control, but dose per fraction was not important. The data were most consistent with the model of delayed accelerated tumor repopulation occurring 30 days after the beginning of treatment with a compensatory dose of 0.73 Gy per day of treatment prolongation. Thus, shortening treatment duration to minimize accelerated repopulation or increasing total dose without adding to late complications have become important areas of investigation. Attempts to improve LRC with altered fractionation have seemed promising in single-institution studies.43,50,89–92
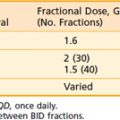
On the basis of such evidence, the RTOG conducted a landmark trial (RTOG-90-03) comparing the leading US altered fractionated regimens for multiple head and neck cancer sites, including oropharynx cancers (60%).93 One thousand and seventy-three patients with primarily advanced SCCs of the head and neck were randomized to four arms:
 Split-course accelerated fractionation (S-AF) with 1.6 Gy b.i.d. to 67.2 Gy during 6 weeks with an intentional 2-week break after 38.4 Gy and an interfraction interval of 6 hours
Split-course accelerated fractionation (S-AF) with 1.6 Gy b.i.d. to 67.2 Gy during 6 weeks with an intentional 2-week break after 38.4 Gy and an interfraction interval of 6 hours Delayed concomitant boost (DCB) with daily morning 1.8 Gy treatments and a 1.5 Gy afternoon concomitant boost for the last 12 days of treatment with a 6-hour interfraction interval and a total dose of 72 Gy during 6 weeks
Delayed concomitant boost (DCB) with daily morning 1.8 Gy treatments and a 1.5 Gy afternoon concomitant boost for the last 12 days of treatment with a 6-hour interfraction interval and a total dose of 72 Gy during 6 weeks Pure hyperfractionation (HF) with 1.2 Gy twice daily with an interfraction interval of 6 hours to a dose of 81.6 Gy per 7 weeks.
Pure hyperfractionation (HF) with 1.2 Gy twice daily with an interfraction interval of 6 hours to a dose of 81.6 Gy per 7 weeks.Horiot et al. reported results of the EORTC 22791 randomized trial comparing an HF regimen of 1.15 cGy b.i.d. (4 to 6 hours between fractions) to 80.5 Gy during 7 weeks versus CF of 1.8 to 2.0 Gy to 70 Gy during 7 to 8 weeks in the treatment of 356 patients with T2 to T3N0 to 1 oropharyngeal cancers excluding the base of tongue.94 With a mean follow-up of about 4 years, HF improved 5-year actuarial LRC compared with CF (59% versus 40%, respectively P = .02) with a trend toward improved survival (38% versus 29%, respectively, P = .08). T3 tumors benefited from HF but T2 lesions did not. More severe acute mucositis occurred in the HF, but did not increase the incidence of treatment interruption. The incidence of grade 2 or 3 late effects was no different between the two arms.
Overgaard et al. reported results of the DAHANCA 6 and 7 trials, which randomized 1476 patients with stage I to IV head and neck cancer to either an accelerated radiotherapy regimen employing 6 weekly fractions of radiation with the standard regimen of 5 weekly fractions in the treatment of patients.95 Both arms were treated to a total dose of 66 to 68 Gy at 2 Gy per fraction (except for T1 glottic tumors, which received a total dose of 62 Gy). An oral dose of the hypoxic radiosensitizer nimorazole (1200 mg/m2) was administered with the first 30 fractions of radiation. The accelerated regimen proved to be superior in terms of 5-year local control (LC; 76% versus 64%, P = .0001), LRC (70% versus 60%, P = .0005) and disease-specific survival (DSS; 73% versus 66%, P = .01). OS was not improved with the shortened course of radiation. Confluent mucositis was significantly higher in the accelerated group (53% versus 33%, P < .0001), but 98% of both arms received the scheduled radiation dose, and late toxicity did not differ between the fractionation groups. Several other randomized trials examining altered fractionation schemes have been reported to date, most of which also demonstrate an improvement in LC and LRC at the expense of increased acute toxicity, but without an impact on OS.96–102
Intensity-Modulated Radiation Therapy as a Surrogate for Altered Fractionation
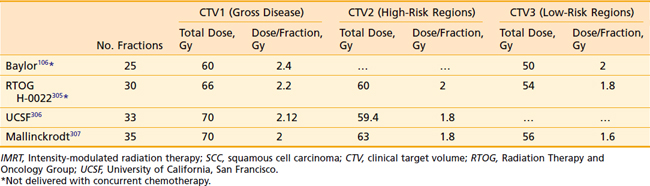
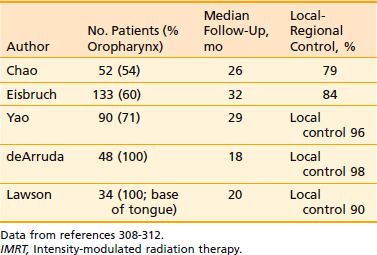
Another issue that arises in the modern era is the role of altered fractionation schemes in the context of IMRT. Although the use of hyperfractionated or accelerated treatment schedules with IMRT have been reported,103–105 because of the expense of time and resources required with IMRT, most radiation oncologists have reverted to treating patients once a day. An alternative to twice-daily radiation is the delivery of a simultaneous integrated boost (SIB), wherein multiple targets are treated simultaneously with different prescribed doses. For example, the primary tumor and gross nodal disease may be treated to 66 Gy in 30 fractions (2.2 Gy/fraction), while at the same time, high-risk areas receive 60 Gy (2 Gy/fraction) and low-risk areas receive 54 Gy (1.8 Gy/fraction). Because of the higher dose per fraction, however, this scheme may result in a greater risk of late morbidity. Alternatively, the gross disease may be treated with standard doses of 1.8 to 2.12 Gy per fraction, while subclinical disease is treated with a lower-than-standard daily dose (see Radiation Therapy Techniques). When this approach is employed, particularly in patients with advanced disease, concurrent chemotherapy should be administered. Common IMRT dose prescriptions are listed in Table 28-7. The optimal dose prescription has not been determined; however, it should be noted that those with the highest doses per fraction, such as that described by Butler et al.,106 should not be delivered with concurrent chemotherapy, due to severe acute mucositis. The use of SIB has not been compared with traditional altered-fractionated radiation in a randomized study, and therefore whether the employment of IMRT with a SIB offers the same benefit as the aforementioned altered fractionation schemes is, at present, unknown. However, the reported experience to date shows excellent LRC using IMRT with SIB with or without concurrent chemotherapy (Table 28-8). The most recent outcomes with IMRT indicate LRC rates of more than 90% in stage III/IV patients treated with primary concurrent chemoradiation.105,107,108 Longer follow-up is required for confirmation.
The Role of Chemotherapy in Advanced Oropharyngeal Cancer
Concurrent Chemoradiotherapy
Patients with advanced tumors of the oropharynx who require extensive surgery with or without total laryngectomy typically have experienced suboptimal results. Owing to the size and location of these tumors, primary surgery can have a significant impact on the functional, psychological, and cosmetic consequences for these patients. The previous standard for organ preservation was established by the Veterans Affairs (VA) Laryngeal Cancer Study Trial109,110 in which induction chemotherapy plus conventionally fractionated EBRT (which allowed preservation of the larynx in 66%) produced survival rates equivalent to those undergoing total laryngectomy with postoperative EBRT. However, recent studies and meta-analyses have demonstrated that a concurrent chemoradiation appears superior to the VA regimen or to EBRT alone and have brought into question the benefit of induction chemotherapy.111–121 Forastiere et al. reported the 5-year results of RTOG 91-11, demonstrating that in patients with larynx cancer requiring total laryngectomy and randomized to (A) conventional fractionated EBRT (70 Gy/7 weeks) alone, (B) the VA regimen, or (C) concurrent cisplatin (100 mg/m2 weeks 1, 4, and 7) with conventional fractionated EBRT (70 Gy/7 weeks), those in arms B and C had superior laryngectomy-free survival compared with conventional RT alone (46.6% and 44.6% versus 33.9%, P = .011), and arm C had a statistically superior 5-year larynx preservation rate (83.6%) compared with both arm A (65.7%, P = .00017) and arm B (70.5%, P = .0029). Moreover, arm C demonstrated a superior 5-year LRC compared with the other two arms. No significant difference in OS was seen.115–117 A number of trials restricted to oropharynx cancers have demonstrated similar findings.
A landmark French intergroup (GORTEC 94-01) phase III randomized trial tested whether the addition of concurrent chemotherapy to conventionally fractionated RT improves outcome compared with conventionally fractionated RT alone for patients with advanced stage oropharynx carcinoma.111–113122 Two hundred and twenty-six patients with stage III (32%) and IV (68%) tumors were randomized to 70 Gy per 7 weeks or 70 Gy with 3 cycles of concurrent carboplatin (70 mg/m2/day × 4) and fluorouracil (5-FU) (600 mg/m2/day × 4) (weeks 1, 4, and 7). The arms were balanced with regard to stage, sex, tumor site, performance status age, and histology. Acute toxicities were increased in the concurrent arm including grade 3 or 4 mucositis (71% versus 39%, P = .005), dermatitis (67% versus 59%, P = .02), and need for feeding tube (36% versus 15%, P = .02) compared with the control arm. However, the incidence of treatment-related mortality (1% [1/109] versus 0%), overall treatment duration (52 versus 50 days, respectively), and treatment interruption ≥3 days (19% versus 16%, respectively) were similar. Severe cervical fibrosis was increased in the combined modality treatment group (12% versus 3%, P = .08, respectively) but the overall rate grade 3 or 4 late toxicity was not statistically significant (56% versus 30%, P = .12). At a median follow-up of 5.5 years, patients in the combined modality arm had a higher 5-year LRC (47.6% versus 24.7%, P = .002), DFS (26.6% versus 14.6%, P = .01) and OS (22.4% versus 15.8%, P = .05) but no difference in the rate of distant metastases (18% versus 17%, respectively). On multivariate analysis, pretreatment hemoglobin levels <125 g/L, stage IV disease, and RT alone were independent prognostic factors of short survival and locoregional failure. The approximately 20% improvement in survival and LRC argues for the addition of concurrent chemotherapy in patients with advanced oropharynx cancer treated with definitive radiation. However, the optimal chemoradiotherapy regimen with regard to fractionation scheme and chemotherapy agents remains to be defined.
In 2006, Bonner et al.123 reported the results of a multicenter, international phase III study investigating the addition of concurrent cetuximab, a monoclonal antibody against the epidermal growth factor receptor (EGFR), to patients receiving definitive radiotherapy for stage III or IV head and neck carcinoma. Patients were treated via standard fractionation, hyperfractionation, or accelerated fractionation with concomitant boost schedules, with or without cetuximab. Sixty percent of the 424 patients enrolled in the study had oropharyngeal primaries. With a median follow-up of 54 months, the addition of cetuximab improved the 3-year LRC rate (47% versus 34%, P < .01), PFS (42% versus 31%, P = .04) and OS (55% versus 45%, P = .05). Although the trial was not sufficiently powered to detect differences among subgroups, the greatest survival benefit was among patients with oropharyngeal cancers, with a median survival of >66 versus 30.3 months. Importantly, although there was an increased incidence of grade 3 to 5 acneiform rash (17% versus 1%, P < .001) and infusion reaction (3% versus 0%, P = .01), the risk of other acute radiation-related side effects such as mucositis (56% versus 52%, P = .44) did not differ significantly between the two groups. Likewise, severe late effects were reported in about 20% of patients in each group. Currently, RTOG 0522 is randomizing patients with advanced head and neck SCC to receive concurrent accelerated radiation and cisplatin, with or without concurrent cetuximab124
Combining Chemotherapy With Altered Fractionated Radiation
As both the addition of concurrent chemotherapy and altered fractionated radiation have been have been shown independently to improve outcome for head and neck cancer patients, the present challenge is to find the optimal chemoradiation regimen(s) that offer high rates of LRC, are not unduly morbid, offer maximal preservation of organ function, and may be integrated with new biologic agents.93,111–113,115–117,123 Multiple phase II and randomized studies have reported possible regimens. The benefit of induction chemotherapy remains to be defined.
Phase II Studies
Harrison reported the results of a phase II trial that treated 82 patients with unresectable head and neck cancer using the DCB technique (70 Gy/6 weeks, b.i.d. RT last 2 weeks) with concurrent cisplatin (100 mg/m2) given every 3 weeks for 2 cycles.125 Adjuvant chemotherapy was given in 68%. Although 40% had initial skull base invasion, the response rate was 94% with 60% complete responses. Oropharynx cancers comprised 23% of all tumors treated. For all patients at a minimal follow-up of 3 years, the 3-year LC, OS, and distant-metastasis-free survival was 58%, 36%, and 58%, respectively. Seventy percent of patients with base of skull invasion were locally controlled. The treatment was reasonably well tolerated. The 3-year LC for oropharynx cancers was 64%. All patients experienced grade 3 acute mucositis usually during the concomitant boost phase. Twenty-four percent required a treatment break, the majority requiring less than one week. Two deaths due to sepsis occurred during treatment. Severe chronic toxicity occurred in three patients with one osteoradionecrosis, one frontal lobe necrosis, and one case of lung toxicity secondary to adjuvant chemotherapy. Given the especially poor prognosis of this group, the LC was good and survival better than expected with such an aggressive chemoradiation program. Our experience in treating advanced oropharyngeal disease was recently updated.126 Forty patients were treated with the above regimen, with either two cycles of cisplatin (100 mg/m2 on weeks 1 and 4) or daily carboplatin (10 to 17.5 mg/m2). Seventy-five percent of patients had T3 or T4 disease, and 57.5% of patients were stage N2 or N3. The 2-year LC, regional control, LRC, freedom from distant metastasis, DFS and OS rates were 81%, 97%, 81%, 74%, 76% and 80%, respectively. Grade 3 toxicity rates were as follows: mucositis, 67.5%; dysphagia, 62.5%, odynophagia, 62.5%. Patients required a median 2.24 days of treatment break. Late toxicities were mainly due to grade 3 to 4 xerostomia (36%, 2.2%, respectively) and grade 3 dysphagia (11.1%). One patient developed osteonecrosis and one patient developed soft tissue necrosis. Of the 80% of patients who had a gastrostomy tube placed before or during treatment, two patients were still dependent on parenteral feeds 5 and 17 months after treatment completion.
A modified version of DCB radiation was reported by Bieri et al. in which a planned total dose of 69.6 Gy was shortened to total of 5.5 weeks and one third of the patients received primarily concurrent cisplatin-based chemotherapy.127 Among the 55 patients with oropharynx carcinoma (76% stage III/IV), LRC at 3 years was 69.5% at a median follow-up of 32 months. Eighty-two percent experienced grade 3 or 4 mucositis. Patients receiving chemotherapy had a greater rate of grade 3 dysphagia (68% versus 25%, P = .003), hospitalization (37% versus 14%, P = .08), and need for nasogastric tube (68% versus 22%, P = .001). Late RTOG grade 3/4 complications occurred in 12% of patients and consisted of laryngeal edema, mucosal necrosis, and mandibular osteoradionecrosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree