The oral cavity is the most common site for squamous cell carcinoma (SCC) of the head and neck, a disease that inflicts substantial morbidity and mortality worldwide.1,2 The primary modality of treatment for both index and recurrent cases is surgical resection, though many patients present with advanced stage disease and require a multidisciplinary approach. A number of issues that impact surgical treatment must be considered, including operative access, need for mandibulectomy, need for neck dissection, and the type of reconstruction. Recovery and rehabilitation often require ancillary services that include dental, prosthodontics, physical therapy, as well as speech and swallow therapy. Patients with adverse pathologic features benefit from postoperative radiation; lymph node extracapsular spread or positive surgical margins in particular suggest need for chemoradiation.3 The current diagnosis and management of oral cavity malignancies are reviewed.
Oral cavity malignancies comprise 30% of all head and neck cancers, with approximately 22,000 cases per year worldwide (excluding lip cancers). Over 95% of oral cavity cancers are SCCs that arise from carcinogen exposure. In the United States the most common subsite is the oral tongue due to smoking. However, in areas such as India, the most common subsite is the buccal mucosa due to the high prevalence of betel nut and other smokeless tobacco consumption.
Tobacco and alcohol are the two most important risk factors associated with the development of oral cavity SCC. For both products, the risk is both dose-dependent and synergistic, with tobacco serving a primary role and alcohol enhancing or promoting its carcinogenic effects. The irritant effects of alcohol induce a chemical burn that increases cell membrane permeability and facilitates introduction of toxic agents. Notably, 75% of oral cavity cancers are found within a zone that encompasses only 10% of the surrounding mucosal surface area.4 This region extends from the anterior floor of mouth, around the lateral tongue, and up to the retromolar trigone and anterior tonsillar fossa. This is most likely due to the natural flow and pooling of contaminated saliva.
Compared to nonsmokers, smoking confers a 1.9-fold risk for males and threefold risk for females, with proportionally escalating risk for increased number of years smoking and number of cigarettes smoked per day.5 Similarly, alcohol alone confers a 1.7-fold risk to males drinking one to two glasses per day compared with nondrinkers, rising to threefold for heavier consumption. Smokers who at least drink two packs per day and those who concurrently drink at least four glasses per day have been found to combinedly harbor a 35-fold increased risk for developing oral cavity SCC compared to normal controls. Separately, individuals who chew smokeless tobacco have a fourfold higher risk of developing an oral cavity malignancy compared with nonusers. Women in India who practice reverse smoking (puffing cigars with the burning end in the mouth) have a 47-fold higher risk of developing malignancies of the hard palate.4
The 5-year overall survival for all oral cavity subsites ranges from 51% to 58%, though for lip cancer it is much higher and approximates 93%. Matched by stage, African Americans, men, and patients older than age 65 have a poorer outcome.6 After treatment, oral cavity patients who continue to smoke have a 40% risk of developing a recurrence or second head and neck malignancy, usually within 3 years.7 Such high rates are associated with continued alcohol and tobacco consumption as well as prior use: it may require up to 20 years of smoking cessation to reduce the risk of developing an oral cancer to that of a nonsmoker.8
Numerous other etiologic factors contribute to oral cavity carcinogenesis and deserve special mention. The use of betel nut (or quid, a formulation that blends betel nut with lime and cured tobacco) is prevalent throughout India and Southeast Asia, acting as a mild stimulant. As an addictive agent, betel nut induces significant mucosal inflammation, eventually leading to chronic and irreversible trismus and submucosal fibrosis, itself a precancerous condition. Poor hygiene, periodontitis, and progressive buccal mucosa thickening ultimately lead to a high incidence of buccal mucosa SCC. Pipe smoking and environmental ultraviolet light exposure have been associated with lower lip malignancies.9 Though human papillomavirus (HPV) is now established as a major causative factor for most oropharyngeal SCC in North America, it is not implicated in oral cavity SCC pathogenesis despite its detection in pathologic specimens.10 This may be due to the lack of crypts favored by HPV or perhaps the stronger causative effects of environmental factors described above. There is an increasing incidence of oral tongue cancer in younger patients who never smoked or had minimal tobacco exposure history. The reason for the change in demographics for this disease is unknown.11
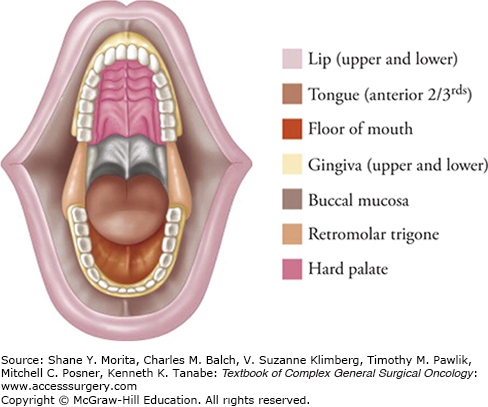
The oral cavity spans from the lip vermillion border to the hard–soft palate junction, the oral tongue–tongue base junction (linea terminalis), and laterally to the anterior tonsillar pillars (Fig. 57-1). The oral cavity is divided into subsites: the lips, upper and lower alveolar ridges, tongue, retromolar trigone, floor of mouth, buccal mucosa, and hard palate. The anatomic relationships between the oral cavity subsites play primary roles in articulation, mastication, and deglutition. As a result, knowledge of the interplay among salivary glands, maxilla, mandible, dentition, and tongue function is imperative for achieving best oncologic and functional outcomes. The fascial planes, neurovascular bundles, and lymphatic drainage pathways also directly affect tumor spread and prognosis: factors such as neck lymph node involvement, perineural invasion, and extracapsular spread have all been shown to alter substantially disease-free and overall survival.
The lips serve as the transition from the facial skin to the specialized internal mucosal membrane, defined by the commissures laterally and the vermillion borders superiorly and inferiorly. They are supported by the sphincter-like orbicularis oris muscle, which is innervated by the branches of cranial nerve (CN) VII. Upper lip sensation is supplied by CNV2 (branches of the infraorbital nerve), while lower lip sensation is provided by CNV3 (mental nerve branches from the inferior alveolar nerve). An embryonic fusion plane that exists at the midline of the upper, but not the lower, lip helps determine lymphatic drainage of disease. Both upper and lower lip lesions tend to drain predominantly to the ipsilateral submandibular lymph node basins, while more midline lower lip lesions may drain to submental lymphatics.
The upper and lower alveolar ridges serve as support for dentition and form part of the jaws. Laterally, the ridges form a gingivobuccal sulcus that transitions to the buccal mucosa. On the lower alveolar ridge, the medial margin borders the floor of mouth, while the posterior margin abuts the retromolar trigone and ascending ramus of the mandible. On the upper alveolar ridge, the medial margin borders the hard palate, while the posterior margin extends to the pterygopalatine arch. The tight adherence of thin mucosa to underlying bone leads to early bone invasion by malignancy relative to other oral cavity subsites.
The oral tongue (anterior two-thirds) is embryologically distinct from the tongue base (posterior one-third) and is separated by the linea terminalis. It is divided at midline by the median fibrous lingual septum and is composed of paired extrinsic and intrinsic tongue musculature. The four paired extrinsic muscles (genioglossus, hyoglossus, styloglossus, and palatoglossus) are anchored to bone and adjust tongue position. The genioglossus is the largest muscle and serves to protrude and depress, while the hyoglossus flattens the tongue toward the hyoid bone, propelling food boluses to the oropharynx. The four paired intrinsic muscles (superior longitudinal, inferior longitudinal, verticalis, and transversus) originate and insert within the tongue and modify its shape, including curling, lengthening, shortening, and rounding the tip. The absence of fascial planes between these muscles enables tumors to penetrate easily and infiltrate among the various muscles.
Motor innervation is supplied by the hypoglossal nerve except for the palatoglossus nerve (CNX). Sensory innervation for the oral tongue is supplied by the lingual nerve (CNV3), which also carries the chorda tympani branches (CNVII) that account for taste. Because CNV3 simultaneously provides sensation for the external auditory canal and tympanic membrane, referred otalgia is a common presenting complaint. Lymphatic drainage importantly depends on the tongue site (Fig. 57-2). The tongue tip primarily drains into the submental (level IA) lymphatics, while the lateral edges drain into ipsilateral levels IB and II. The anterolateral tongue has lymphatic drainage to level III lymph nodes, thus explaining the presence of mid-neck metastasis among some patients with primary tumors arising in this location. In contrast to base of tongue lymphatics, there is seldom passage across midline: the contralateral neck generally does not require surgical management unless the tumor is near midline or at the tip or if there are clinically evident lymph node metastases.
The retromolar trigone is a small triangular mucosal space bordered by the last molar, the ascending mandibular ramus, and the maxillary tuberosity up toward the coronoid process. It connects freely with the buccal mucosa and anterior tonsillar pillar. Similar to the alveolar ridge, the thin adherent mucosa is easily penetrated by tumors and bony invasion is common. Sensation is provided by the lesser palatine nerve and CNIX. Patients may present with referred otalgia from CNIX impingement, or lower lip paresthesias from inferior alveolar nerve involvement. Lymphatics preferentially drain to level II neck basins.
This space is located between the lower alveolar ridge and oral tongue, and bisected by the lingual frenulum. Wharton’s ducts (submandibular ducts) pierce the floor of mouth and drain saliva on either side of the frenulum. Underlying muscular support is provided by the mylohyoid and hyoglossus muscles, between which the sublingual glands sit, as well as the genioglossus muscle. The hypoglossal and lingual nerves travel through this region and are commonly involved by tumor. The lingual nerve provides sensation. Lymphatic drainage tends to be bilateral (levels IA and IB) for anterior floor of mouth lesions and ipsilateral (level II) for posterior lesions.
The buccal mucosa is a mucosal surface situated between the posterior lips, the alveolar ridge medially, and the pterygomandibular raphe posteriorly. The layers underlying the mucosa are the pharyngobasilar fascia, the buccinator fat pad, the buccinator muscle (innervated by CNVII), and finally the subcutaneous tissue and cheek skin. Sensation is supplied by CNV2 and CNV3, and lymphatic drainage is to level IB. Notably, the lack of bony or fascial planes leads to relatively fast and uninhibited tumor spread, making buccal mucosal cancer more aggressive with poorer prognosis.12,13
The hard palate is created by the palatine process of the maxilla and the horizontal plate of the palatine bone. The junction of the hard and soft palates harbor the foramina through which the greater and lesser palatine nerves and vessels travel. The incisive foramen is posterior to the maxillary incisors and conveys the nasopalatine vessels and nerve. Lesions spread easily through these foramina into the nasal vault or maxillary sinus. Also challenging to address is lymphatic spread: posterior hard palate lesions that extend to the soft palate drain to level II but also to retropharyngeal lymph node basins, which are difficult to treat surgically.
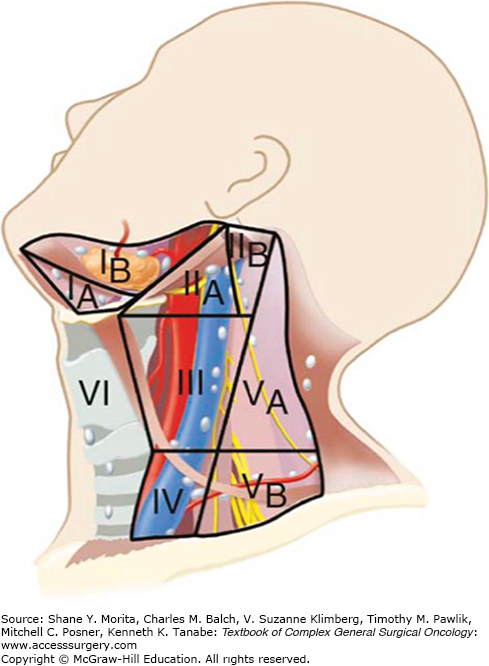
The head and neck lymphatic network is divided into superficial and deep layers separated by the deep cervical fascia. The superficial lymphatics drain into suboccipital, preauricular and postauricular, and external jugular lymph nodes. Deep cervical lymph nodes primarily drain the mucosa of the upper aerodigestive tract. These lymph nodes include the submental, prevascular facial, and submandibular group located in the submental and submandibular triangles. Deep jugular lymph nodes include the jugulodigastric, jugulo-omohyoid, and supraclavicular group of lymph nodes adjacent to the internal jugular vein. Lymph nodes in the posterior triangle of the neck include the accessory chain located along the spinal accessory nerve and the transverse cervical chain in the floor of the posterior triangle.
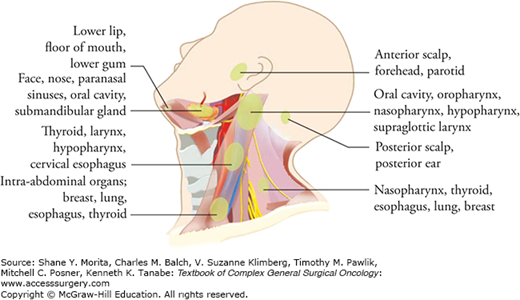
The standardized description of regional node metastasis divides the lymph nodes in the lateral aspect of the neck into five nodal groups or levels (Fig. 57-2). In addition, lymph nodes in the central compartment of the neck are assigned level VI, and those in the anterior superior mediastinum are assigned level VII. Each subgroup of lymph nodes serve as the primary echelon from a specific site in the head and neck region. Thus, the location of a palpable metastatic lymph node often indicates the likely source of a primary tumor (Fig. 57-3).
The anatomic complexity, mix of structures, and environmental agents that pass through the oral cavity all contribute to a diversity of benign and malignant lesions that defy easy identification. Irritation from dental prostheses, immunosuppressive conditions, and inflammatory changes may also make diagnosis challenging. In such cases, close surveillance and judicious biopsy are necessary to properly manage any suspicious abnormality. The most common premalignant and malignant lesions are discussed below.
Leukoplakia is a common finding and is defined as a white keratotic plaque that cannot be removed from underlying tissue. As a clinical term without histologic definition, it is better classified by the associated amount of dysplasia, which is abnormal mucosal epithelium harboring features of increased nuclear:cytoplasm (N:C) ratio, increased mitoses, and loss of intracellular adherence. Although only 1% to 3% of leukoplakic lesions have dysplasia, any level of dysplasia increases the risk of progression to malignancy by a factor of seven. A longitudinal study of over 600 cases of leukoplakia found that 86% disappeared, improved, or did not change, while 6% progressed to SCC.14 Erythroplakia appears as a persistent red hyperkeratotic plaque, and carries a substantially higher risk of malignancy. In one case series, more than 90% of such lesions were identified with invasive SCC, carcinoma in situ, or severe dysplasia.15
Lichen planus is recognized via a lacy pattern with white striae, with atrophic (red, smooth appearance) and erosive (depressed margins with fibrinous exudate) subtypes. Histologically they contain T-cell lymphocyte infiltration, and are estimated to have a 1% chance of malignant transformation over 10 years.16
Necrotizing sialometaplasia is often mistaken for malignancy but is a benign entity. It is commonly seen as a grossly ulcerative lesion at the junction of the hard and soft palate. Such lesions are due to pressure injury or irritation to minor salivary glands, usually from an ill-fitting denture or other prosthesis.
Several variants to conventional SCC exist with differing clinical features. Basaloid SCC is a high-grade variant with a 64% incidence of neck lymphadenopathy and a 44% incidence of distant metastases.17 Verrucous carcinoma, in contrast, is a low-grade variant and commonly seen in the buccal mucosa as an exophytic lesion. Its behavior tends to be locally aggressive but with a low rate of regional or distant metastases. Finally, sarcomatoid SCC (represented by spindle cells interwoven within squamous cells) is moderately aggressive, with a documented 37% rate of metastases.18
Numerous less common malignancies also present within the oral cavity and must be considered in the differential diagnosis. The most commonly found salivary gland malignancies in the oral cavity include mucoepidermoid carcinoma, adenoid cystic carcinoma, polymorphous low-grade adenocarcinoma, and adenocarcinoma. Though minor salivary glands are found throughout the oral cavity, salivary malignancies in the oral cavity are usually found in the palate. Sarcomas may arise from the mandible or hard palate, and include osteosarcoma, chondrosarcomas, malignant fibrous histiosarcoma, rhabdomyosarcoma, and liposarcoma. Mucosal melanoma is relatively rare but highly aggressive, often presenting as a pigmented lesion that rapidly metastasizes with a poor prognosis.
All head and neck patients should undergo a comprehensive history and physical examination. Specific questions should be asked about mouth ulceration, dysphagia, odynophagia, dysarthria, facial numbness, weight loss, referred otalgia, and trismus. Changes in a previously well-fitted denture should also raise suspicion.19 An accurate history about tobacco and alcohol use should be solicited as well as the patient’s functional status and comorbidities. These factors play an important role for devising an appropriate treatment plan and the patient’s likelihood of completing it.
The physical exam evaluates the size and character of the oral cavity lesion, including its thickness, its degree of infiltration (endophytic or exophytic), and its potential involvement with adjacent structures and pathways of spread. Firm palpation should be performed to assess degree of fixation or invasion to the mandible or maxilla. A lesion that bleeds easily should raise suspicion of malignancy. Beyond the index mass, cranial nerve deficits or paresthesias should be documented, regional lymphadenopathy palpated, and synchronous cancers sought. Grossly compromised teeth that are unlikely to survive radiation therapy should be evaluated for extraction. Conversely, loose teeth at the tumor site should be retained, as premature removal opens dental sockets and facilitates implantation into the mandibular canal.
Surgical access to the lesion should be considered, as well as potential pedicled and microvascular reconstruction options. Any suspicious lesion should be biopsied to confirm malignancy. A fine-needle aspiration of suspicious neck lymphadenopathy also is useful for diagnosis and staging.
The use of complementary imaging modalities is a crucial adjunct to the physical exam in evaluating oral cavity lesions for staging and treatment planning, especially with regards to bone invasion. To evaluate the primary lesion, a Dentascan® and contrast soft tissue computed tomography (CT) scan are indicated. Dental CT scans provide finer detail and enable three-dimensional modeling of the bony regions that is useful for reconstruction. A standard CT scan is also valuable for evaluating lesions that arise from the palate or alveolar ridge, capturing tumor extension into adjacent areas superiorly or posteriorly. Coronal cuts provide an excellent sense of extension through palatal foramina as well as mandibular height when evaluating the need for marginal or segmental mandibulectomy. While CT is good for cortical bone involvement, magnetic resonance imaging (MRI) is preferred for medullary bone extension as well as soft tissue invasion (such as into the masticator or parapharyngeal spaces).
To evaluate the neck for regional lymph node involvement a contrast CT scan should be performed. Enlarged nodes, rounded nodes, and the presence of central necrosis are suggestive of metastatic spread. For the evaluation of distant metastases, positron emission tomography/computed tomography (PET/CT) may be useful for advanced cancer stage. If this shows evidence of PET uptake in the lungs, then a chest CT should be done for further evaluation.
After clinical examination and appropriate imaging and biopsy it is usually possible to accurately stage the patient without the need for any further endoscopy. In the past, panendoscopy (i.e., direct laryngoscopy, bronchoscopy, and esophagoscopy) was routinely performed to exclude the possibility of a synchronous cancer. However, with advances in imaging and flexible endoscopy, most institutions now reserve bronchoscopy and esophagoscopy for cases with worrisome imaging or with symptoms such as hemoptysis or odynophagia.
The current American Joint Committee on Cancer (AJCC) staging system for oral cavity cancer is shown in Table 57-1. The T stage is largely defined by the size of the lesion. For lip cancer, T4 disease specifies tumor involving cortical bone, the inferior alveolar nerve, the floor of mouth, or the skin of the face. For other oral cavity subsites, T4a disease signifies surgically resectable disease, with involvement of adjacent structures or the skin of the face. T4b disease, however, suggests massive, surgically unresectable disease, with involvement of the masticator space, pterygoid plates, skull base, or internal carotid artery encasement. The N stage is defined by the size of the lymph nodes as well as the number of lymph nodes involved.
AJCC Staging System for Lip and Oral Cavity Cancera
| T | Primary tumor |
|---|---|
| T0 | No evidence of primary tumor. |
| T1 | Tumor ≤2 cm in greatest dimension. |
| T2 | Tumor >2 cm but ≤4 cm in greatest dimension. |
| T3 | Tumor >4 cm in greatest dimension. |
| T4a | (Lip) Tumor invades through cortical bone, inferior alveolar nerve, floor of mouth, or skin of face, that is, chin or nose. (Oral cavity) Tumor invades adjacent structures only (e.g., through cortical bone (mandible or maxilla) into deep (extrinsic) muscle of tongue (genioglossus, hyoglossus, palatoglossus, and styloglossus), maxillary sinus, or skin of face). |
| T4b | Tumor invades masticator space, pterygoid plates, or skull base and/or encases internal carotid artery. |
| N | Regional lymph nodes |
| N0 | No regional lymph node metastasis. |
| N1 | Metastasis in a single ipsilateral lymph node, ≤3 cm in greatest dimension. |
| N2a | Metastasis in single ipsilateral lymph node, >3 cm but ≤6 cm in greatest dimension. |
| N2b | Metastases in multiple ipsilateral lymph nodes, none >6 cm in greatest dimension. |
| N2c | Metastases in bilateral or contralateral lymph nodes, none >6 cm in greatest dimension. |
| N3 | Metastasis in a lymph node >6 cm in greatest dimension. |
| M | Distant metastasis |
| M0 | No distant metastasis. |
| M1 | Distant metastasis. |
Patients with oral cavity cancer often require a multidisciplinary approach to their care. A dental evaluation is important, especially for patients requiring radiation therapy. Dental prophylaxis with fluoride treatment, extraction of nonsalvageable teeth, and restoration of viable ones is necessary to prevent subsequent wound breakdown, radiation treatment breaks, and eventual osteoradionecrosis (ORN). Prosthodontics evaluation is necessary for patients requiring a maxillectomy in order to construct a suitable denture or obturator. A nutrition consult addresses the need for a gastrostomy tube and risks of malnutrition, which may lead to impaired wound healing, extended hospitalization, or failure to complete the treatment course. Similarly, a preoperative appointment with speech-language pathology is important for preparing the patient, who may face speech and swallowing impairment postoperatively and will need long-term rehabilitation. Added difficulties with care are to be expected when a temporary tracheostomy is planned. Finally, anticipated large defects require consultation with a microvascular plastic surgeon to determine the optimal reconstruction approach.
The various specialties involved with the management of oral cavity cancer patients highlight the importance of cases being presented at a multidisciplinary tumor board. In addition to the advantage of having radiology and pathology services aid in treatment planning, a tumor board consolidates the opinions of different experts to facilitate consensus. Care outside this tertiary multidisciplinary setting has been shown to produce incorrect staging of disease, radiation treatment breaks, geographic radiation misses, early chemoradiation termination, aborted surgical procedures, lack of adherence to established National Comprehensive Cancer Network (NCCN) guidelines, and missed opportunities for patients to enter into innovative clinical trials. Crucially, it has been demonstrated that such miscues collectively lead to decreased survival.20–22 The concept of package time, defined as the period from surgery to completion of radiation therapy, has been demonstrated as important in relation to outcomes. In head and neck cancer, a total treatment package time of less than 100 days is associated with improved locoregional control and survival.23 Such findings support the importance of an operative approach that minimizes risk of complications that delay radiation, and incorporating a radiation protocol that minimizes the risk of treatment breaks or termination.
While the TNM staging system accurately predicts overall survival, there are many other pathological factors which have been shown to affect outcome. Besides grade, the most important histologic feature in oral cavity SCC is depth of invasion. Lesions that are thin and superficially invasive have a low risk of regional lymph node metastasis and are highly curable. In contrast, thicker lesions pose a high risk of occult neck lymphadenopathy and merit neck dissection (Fig. 57-4). Current evidence supports a thickness of 4 mm or greater to be an independent predictor of cervical metastasis.24,25
Figure 57-4
The incidence of lymph node metastasis and survival in relation to the thickness of the primary lesions for T1 and T2 oral cavity squamous cell carcinoma. (Reproduced with permission from Shah JP, Patel SG, Singh B.Jatin Shah’s Head and Neck Surgery and Oncology.4th ed. Philadelphia, PA: Elsevier/Mosby; 2012.).
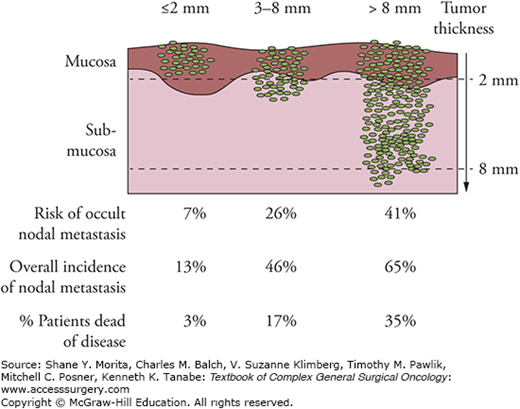
Therefore such lesions warrant elective neck dissection, even if nothing is detected on examination or imaging. While it is not practical to know the exact depth prior to resection, palpation of the primary lesion can provide a gross indicator of depth; staging the neck dissection after initial tumor resection (and surgical pathology results are confirmed) may be feasible. Alternatively the primary tumor may be resected and the depth of invasion measured on frozen section thus guiding decision making with respect to elective neck dissection.
Other unfavorable factors include perineural invasion, infiltrative borders (compared to pushing borders), lymphovascular invasion, and bone invasion. Positive or close (<5 mm) margins are also a poor prognostic factor. Ganly et al26 examined clinicopathologic features affecting outcome in 216 patients with early-stage oral cavity SCC. On multivariate analysis, occult neck metastasis was the main independent predictor of overall, disease-free, and recurrence-free survival (RFS); its presence increased mortality by a factor of five. Additionally, positive margins were a predictor for decreased RFS, while greater than 2 mm depth of invasion was the main predictor for failure in the neck.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree