27 Cancer of the Nasopharynx
Epidemiology
Nasopharyngeal carcinoma (NPC) is uncommon in most parts of the world. The incidence is highest among people from southern China, particularly those originating from Kwantung Province, followed by the Chinese mixed populations of Southeast Asia and indigenous populations in Alaska.1,2 It is of intermediate incidence in North Africa and in the Philippines and it is rare among whites and Japanese. The age-adjusted incidence rate (per 100,000 population per year) ranges from 28.8 in Hong Kong, 17.2 in indigenous populations in Alaska, 16.8 in Singapore, 4.6 in the Philippines, 2.8 in Algeria, and 0.6 in the United States and Japan.3,4 The peak incidence occurs in the fourth and fifth decade of life, and the male/female ratio is 2 : 1 to 3 : 1.
Causal Factors
The cause of NPC is most likely multifactorial. Current epidemiologic and experimental data suggest at least three major causal factors: (1) viral, (2) genetic, and (3) environmental. Epstein-Barr virus (EBV) has long been associated with NPC. Antibody titers to EBV have been found to be elevated in NPC patients regardless of their ethnic and geographic origin. The EBV genome has been demonstrated by nucleic acid hybridization in biopsies from NPC. Recent studies show that EBV nuclear antigen was expressed in nearly all cases and that latent membrane protein was expressed in approximately two-thirds of cases of EBV-positive NPCs.5 Using real-time polymerase chain reaction technology, Lo6 was able to detect circulating EBV deoxyribonucleic acid (DNA) in 96% of NPC patients in Hong Kong. The high incidence of NPC among southern Chinese and southeast Asian populations of southern Chinese descent suggests a genetically determined susceptibility. Highly significant differences in histocompatibility human leukocyte antigen (HLA) patterns have been found between Chinese NPC patients and control subjects. There is a significant increase of HLA-A2 and HLA-B-SIN2 in Chinese patients.7 However, the high-risk HLA pattern is not present in all NPC patients and such a pattern is present in some individuals with no NPC. The decreased incidence of NPC in successive generations of Chinese-born populations in the United States8 suggests a role for environmental factors in the causal factors of this disease. Various environmental factors such as poor ventilation, occupational exposures to smoke or dusts, and diet have been implicated. The ingestion of salted fish during early childhood has been suggested as the most important environmental factor among the southern Chinese with NPC.2,9,10 Dimethylnitrosamine, a carcinogen found in salted fish, has been shown to induce carcinoma in the upper respiratory tract of rats.9
Anatomy
Anteriorly, the nasopharynx is in continuity with the nasal cavity through the posterior choanae, which are separated by the nasal septum. The posterior wall lies at the level of the first two cervical vertebrae and is continuous with the roof. The roof is formed by the basisphenoid, basiocciput, and the anterior arch of the atlas. The lymphoid tissue in this area forms the pharyngeal tonsil (adenoids) (Fig. 27-1). Each of the lateral walls contains the eustachian tube orifice, which is surrounded by the torus tubarius (see Fig. 27-1), a prominence in the cartilaginous portion of the tube. Behind the torus tubarius is a recess called the Rosenmüller fossa (see Fig. 27-1 and 27-2). The lateral walls, including the Rosenmüller fossa, are the most common sites of origin of NPC. The floor of the nasopharynx consists of the upper surface of the soft palate and communicates with the oropharynx via the pharyngeal isthmus. The isthmus is bounded by the uvula anteriorly, the palatopharyngeal arches laterally, and the posterior pharyngeal wall posteriorly.
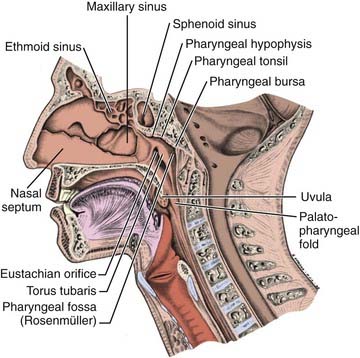
FIGURE 27-1 • Mid-sagittal section of the head showing the nasopharynx and related structures.
(From MacComb WS, Fletcher GH: Cancer of the head and neck, Baltimore, 1967, Williams & Wilkins, p 154.)
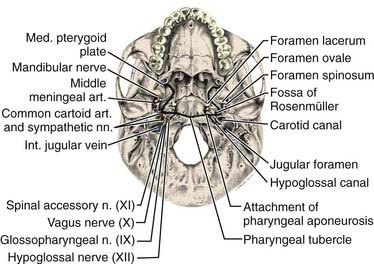
The pharyngeal fascia of the posterior and lateral walls of the nasopharynx is attached to the pharyngeal tubercle on the basiocciput just in front of the foramen magnum. It extends laterally on each side to the ridge on the undersurface of the petrous pyramid, just in front of the carotid canal and anteriorly to the apex of the petrous portion of the temporal bone, and then to the posterior border of the medial pterygoid plate. This fascia is continuous with the fibrous tissue occupying the foramen lacerum, which is separated from the middle cranial fossa only by fibrocartilaginous tissue. Five other foramina are adjacent to the wall of the nasopharynx: the foramen ovale, the foramen spongiosum, the carotid canal, the jugular foramen, and the hypoglossal canal (Fig. 27-3). The foramen lacerum and the foramen ovale constitute the petrosphenoidal crossway, providing an easy pathway into the cranium, and are in close anatomic relationship to the cavernous sinus and hence the II, III, IV, and VI cranial nerves, and the trigeminal ganglion of the fifth nerve and its branches.
Anterior to the eustachian tube, the lateral wall of the nasopharynx is in close relation with the maxillopharyngeal space, which is limited laterally by the ascending ramus of the mandible. In this space is the mandibular nerve descending from the foramen ovale. Posterior to the eustachian tube, Rosenmüller fossa is in close relationship with the lateral pharyngeal or retroparotidian space. The lateral pharyngeal or retroparotidian space is limited anteriorly by the parotid gland and the styloid process and its muscles, posteriorly by the transverse process of the first cervical vertebra, and laterally by the sternocleidomastoid muscle. It contains the lateral pharyngeal nodes, including the lateral retropharyngeal node of Rouvière; the internal carotid artery; the internal jugular vein; and the glossopharyngeal, vagus, spinal accessory, and hypoglossal nerves, as well as the cervical sympathetic nerves as they emerge from the base of skull (Fig. 27-4).
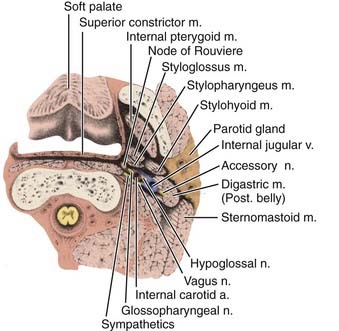
FIGURE 27-4 • Poststyloid portion of the lateral pharyngeal space and its contents.
(From MacComb WS, Fletcher GH: Cancer of the head and neck, Baltimore, 1967, Williams & Wilkins, p 157.)
Lymphatic Drainage
The nasopharynx has a rich lymphatic plexus, particularly in the roof and in the posterior and lateral walls. The lymphatics from the nasopharynx have three major pathways (Fig. 27-5):
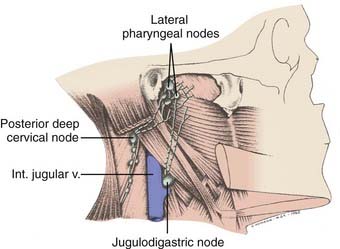
FIGURE 27-5 • Major lymphatic drainage of the nasopharynx.
(From MacComb WS, Fletcher GH: Cancer of the head and neck, Baltimore, 1967, Williams & Wilkins, p 158.)
Pathologic Conditions
The majority of malignant nasopharyngeal tumors (80% to 99%) arise from the epithelium and should be considered as variants of squamous cell carcinoma. According to the most commonly used World Health Organization (WHO) classifications, carcinomas of the nasopharynx are classified into three histologic types: (1) squamous cell carcinoma, (2) nonkeratinizing carcinoma, and (3) undifferentiated carcinoma.11 In 1991 the WHO reclassified NPC into the following histologic subtypes: (1) keratinizing squamous cell carcinoma, (2a) nonkeratinizing carcinoma, and (2b) undifferentiated carcinoma.12 The histologic distinctions among these three types are by no means sharp. Some lesions share intermediate features and some are histologic hybrids. The term lymphoepithelial carcinoma or lymphoepithelioma is used to describe nonkeratinizing and undifferentiated NPCs in which numerous lymphocytes are found among the tumor cells. The distribution of WHO histopathologic types varies with geography, race, and national origin.13 Asians have the highest proportion of WHO types 2 and 3. In Hong Kong, approximately 3% are type 1, 9% are type 2, and 88% are type 3.14 In North America, approximately 20% of NPCs are type 1, 10% are type 2, and 70% are type 3.15 WHO type 1 includes 75% of the U.S. NPC cases and are found most often in U.S.-born, non-Hispanic whites. WHO types 2 and 3 compose the remaining 25% and are more common in Asians. Other uncommon malignant tumors found in the nasopharynx include adenocarcinoma, lymphoma, plasmacytoma, melanoma, and sarcomas.
Clinical Presentation
A neck mass is the most frequent presenting symptom and sign in carcinoma of the nasopharynx.16 Other common symptoms include epistaxis, decreased hearing, nasal obstruction, pain, and cranial nerve deficits. Cervical lymphadenopathy is present in 87% of patients.17 Typically, a mass is visible in the upper posterior neck and palpable beneath the superior aspect of the sternocleidomastoid muscle at the tip of the mastoid. This is due to metastasis in the parapharyngeal or superior posterior cervical nodes of the spinal accessory chain or both. Bilateral cervical lymph node metastases occur in approximately 50% of cases. The most frequently involved nodes are the upper posterior cervical, parapharyngeal, and jugulodigastric nodes. The midjugular and midposterior cervical nodes are the next most commonly involved, followed by the lower jugular and supraclavicular nodes. The submental nodes and occipital nodes are occasionally involved, usually in the presence of massive cervical lymphadenopathy in the more commonly involved neck sites. Rarely, patients present with metastatic preauricular nodes. Nasal voice occurs as a consequence of nasal obstruction, loss of nasopharyngeal resonance, and mechanical interference with normal movement of the soft palate. Nasal discharge, epistaxis, and coughing up of blood-tinged sputum from postnasal drip are symptoms due to local tumor effect. Impaired hearing of the conductive type, usually unilateral with or without tinnitus and serous otitis media, occurs as a result of obstruction, usually by compression rather than direct extension, of the eustachian tube orifice by the primary tumor.
Distant metastasis at presentation is detected in approximately 3% of cases.18 The bones, lungs, and liver are the most common distant metastatic sites.
Routes of Spread
Local Extension
Superior and Posterior
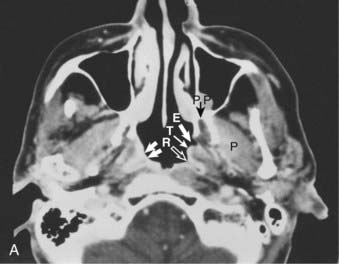
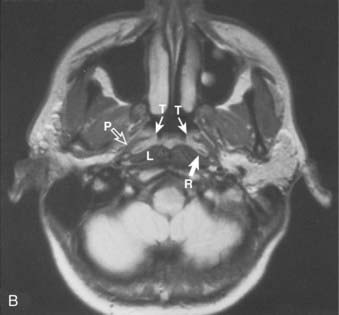
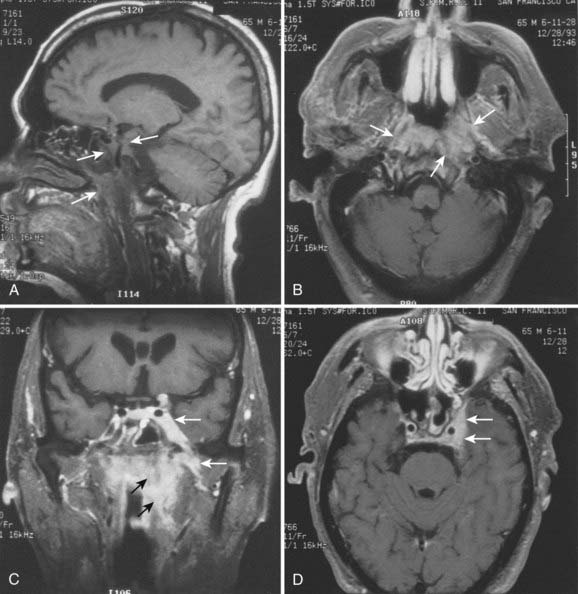
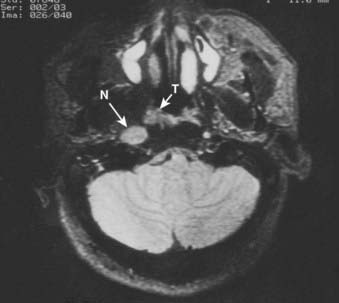
Superiorly and posteriorly, the tumor can directly invade the base of the skull, the sphenoid sinus, and the clivus. The foramen lacerum, located directly above Rosenmüller fossa, is a weak spot in the base of the skull, through which the tumor can gain access into the cavernous sinus and the middle cranial fossa and invade cranial nerves II to VI. Tumor may also invade through the foramen ovale into the middle cranial fossa, the petrous part of the temporal bone, and the cavernous sinus. Invasion of the prevertebral (longus capitus) muscles posteriorly is commonly seen on magnetic resonance imaging (MRI) scans (Fig. 27-6).
Lateral
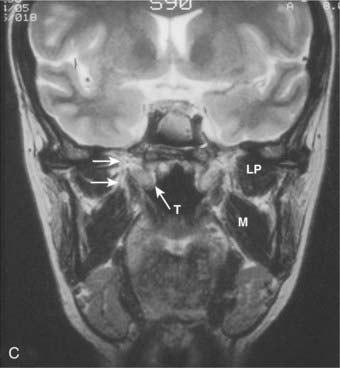
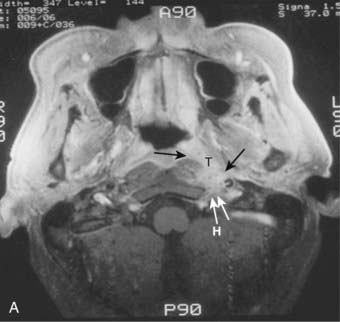
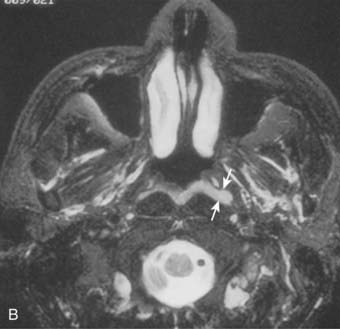
Lateral spread into the lateral parapharyngeal space and invasion of the levator and tensor veli palatini muscles occur early and are commonly seen on MRI scans (see Fig. 27-6 and Fig. 27-7). Invasion of the pterygoid muscles occurs in more advanced disease. Direct tumor extension or lateral retropharyngeal lymph node metastasis in the parapharyngeal space can lead to compression or invasion of cranial nerve XII as it exits through the hypoglossal canal, cranial nerves IX to XI as they emerge from the jugular foramen and the cervical sympathetic nerves. Compression or direct invasion of the internal carotid artery can also occur in advanced disease. Through the eustachian tube, tumor can directly invade the middle ear.
Lymphatic Spread
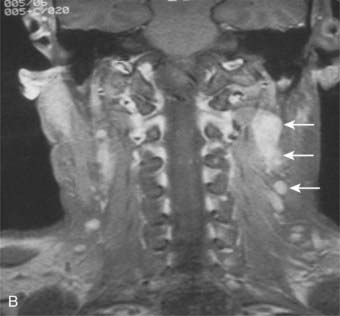
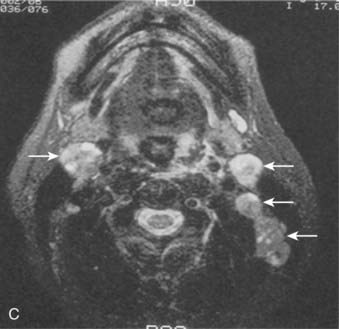
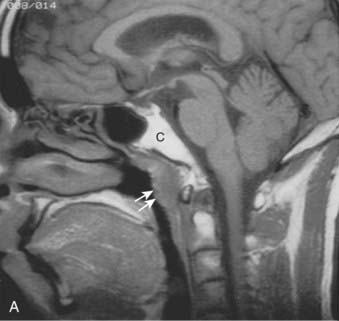
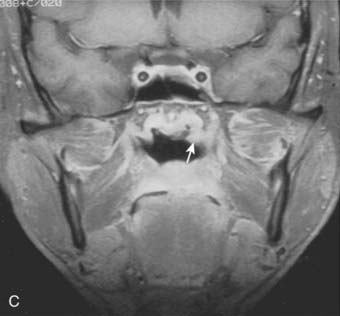
Lymphatic spread to the ipsilateral nodes is common and is present in 85% to 90% of cases.17 Bilateral spread occurs in approximately 50% of cases. Metastasis to the contralateral nodes only is uncommon. The distribution of clinically palpable nodes at diagnosis is shown in Fig. 27-8. Spread to the lateral and posterior retropharyngeal lymph nodes occurs early and is frequently seen on MRI or computed tomography (CT) scans, although the nodes are not palpable (Fig. 27-9). Metastasis to the jugulodigastric and superior posterior cervical nodes is also common. From these first echelon nodes, further metastasis to the midjugular and posterior cervical, lower jugular, and posterior cervical and supraclavicular nodes can occur. Occasionally, spread to the submental and occipital nodes occurs as a result of lymphatic obstruction caused by extensive cervical lymphadenopathy. Metastasis to the mediastinal lymph nodes may occur when supraclavicular lymphadenopathy is present, and occasionally there is metastasis to the axillary nodes as well. Distant lymph node metastasis is often reported in autopsy series, but is rarely apparent clinically at diagnosis.
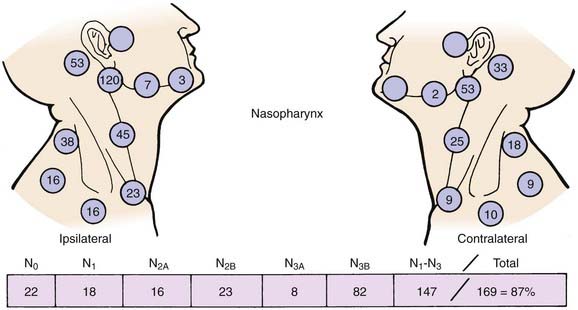
FIGURE 27-8 • Distribution of clinically palpable cervical lymph nodes at diagnosis.
(From Fletcher GH: Textbook of radiotherapy, ed 3, Philadelphia, 1980, Lea & Febiger, p 260.)
Hematogeneous Spread
Distant metastasis is present in 3% of the cases at diagnosis and may occur in 18% to 50% or more of cases during the course of the disease.19–23 An 80% incidence rate has been reported in a small autopsy series.24 The incidence of distant metastasis is highest in patients with advanced neck node metastasis,19,25–27particularly in the low neck.28,29 Bone is the most common distant metastatic site followed by the lungs and liver.
Diagnostic and Staging Studies
The following pretreatment diagnostic evaluations are recommended:
Both MRI and CT scans are useful in diagnostic imaging of the nasopharynx, as well as in radiotherapy treatment planning. However, MRI is the imaging technique of choice in the staging evaluation of NPC.30–32 Because the current T classification system requires a search for tumor invasion into the soft tissue, such as parapharyngeal space, and bony structures, MRI may be necessary for proper staging because CT has limitations in accurately defining the tumor extent in these regions.33 MRI is capable of multiplanar display of tumor extent and is superior to CT scans in delineating muscle, other soft tissue involvement, and evaluating the skull base (see Fig. 27-6, Fig. 27-7, and Fig. 27-10).33–35 In post-treatment follow-up examinations, it has the ability to differentiate radiation fibrosis from persistent or recurrent tumor based on T2-weighted signal intensities and with gadolinium enhancement and fat suppression.36 MRI and CT scans can also detect lymph node metastasis that may not be evident on clinical examination37 (see Fig. 27-9). CT scan is superior to MRI in the detection of early bone invasion.32,34 CT scan with bone windows is useful to demonstrate the extent of invasion of the base of the skull or cervical vertebrae, although MRI is preferable. The major limitation of CT is its poor tissue differentiation, which may be problematic in the evaluation of patients after radiotherapy.35
PET CT scanning has been used more frequently recently in the staging of nasopharyngeal cancer patients. Preliminary data suggest that the standardized uptake value (SUV) may be predictive for failure at distant sites.38,39 PET CT is now commonly used in lieu of conventional staging with CT, bone scans, and ultrasound studies, and appears to be at least as sensitive, but large comparative studies of these staging modalities are not available.40,41
The close association between EBV and NPC, regardless of geographic and ethnic background, has provided a tumor marker for the diagnosis of this disease. Both IgA anti-VCA and IgG anti-early antigen (EA) antibodies are sensitive for the diagnosis of NPC, although IgA anti-VCA is more specific.15 Elevated IgA antibody titers have been reported in more than 90% of untreated patients with NPC from Hong Kong, East Africa, and California.42–44 Positive IgA anti-VCA and IgG anti-EA serologies are primarily associated with WHO type 2 and type 3 NPC. Elevated IgA anti-VCA antibody titers were detected in 82%, and IgG anti-EA antibody titers were detected in 86% of patients with nonkeratinizing carcinoma and undifferentiated carcinomas, but in only 16% and 35%, respectively, of patients with squamous cell carcinomas in a North American study.15 Elevated serum IgA anti-VCA antibodies may be demonstrated in patients months before the onset of symptoms and may serve as a screening test in high-risk patients.45,46 Quantification of plasma EBV DNA can be useful at baseline as it appears to add to the prediction of outcome and levels over time can be used as a post-treatment surveillance tool.47,48
Staging Classification
Various staging systems have been formulated for NPC.9,49–55 The most widely used systems in the English literature are the American Joint Committee on Cancer (AJCC), Union International Cancer Centre (UICC), and Ho systems. The latest versions of the AJCC and UICC (2002) systems are virtually identical. Table 27-1 shows the AJCC and Ho systems currently in use. Each system has its limitations. In the AJCC and UICC systems, the designation of T1 or T2, which is based on the extent of involvement within the nasopharynx, is not meaningful and carries no prognostic significance.53,56 In the 2002 version of the AJCC staging system with regard to T2 lesions, it is divided into with or without parapharyngeal involvement, which has prognostic significance.57 Those with parapharyngeal space involvement are at higher risk for local and regional recurrence, as well as a high rate of distant metastases.58 Cranial nerve involvement has been shown to carry a worse prognosis than base of the skull involvement27,28,51,59; however, both are included in the T4 classification in the AJCC (2002) and the UICC systems and the T3 in Ho’s system. With respect to N-stage classification, the Ho system, which is based on the level or location of neck node involvement, appears to be superior to the AJCC and the UICC system, which are based on size, number, and laterality of the lymph nodes involved.10,28,53 Because of these limitations, modifications of both Ho’s and AJCC classification have been proposed and are shown in Table 27-1. Retrospective comparison of the 1997 or the 2002 AJCC with the 1988 AJCC classification in patients staged with CT or MRI scans suggest a more even stage distribution and better correlation with prognosis with the 1997 or the 2002 AJCC classification.60 In the 2010 AJCC staging manual, T1 patients include those who have disease confined to the nasopharynx, or tumor extending to oropharynx and/or nasal cavity without parapharyngeal extension. Tumor with parapharyngeal extension is now staged as T2. There is no longer a subdivision between T2a and T2b disease. The former T2a disease is now down staged as T1 disease in this new staging system.61 With regard to N staging, because retropharyngeal nodes are the first echelons of nodal metastases,62 retropharyngeal lymph node involvement independent of laterality and without cervical lymph node involvement has been proposed as stage N1.61 The adequacy of these new modified staging classifications will require further clinical confirmation.
Standard Therapeutic Approaches
Although surgery is not used in the treatment of the primary tumor, radical neck dissection is the treatment of choice for resectable neck disease that is persistent or recurrent after radiotherapy.63,64 Surgery has also been used to salvage selected patients with locally recurrent disease.65–68
Concurrent chemoradiotherapy using cisplatin (CDDP)-based chemotherapy is the current standard for patients with NPC with stage III or IV (nonmetastatic) disease. Because of the known chemosensitivity of NPC, a series of randomized controlled trials of chemoradiotherapy versus radiotherapy conducted in the 1990s and early 2000s demonstrated that in patients with advanced local or regional disease, the addition of concurrent chemotherapy to radiation was associated with a statistically significant and clinically meaningful survival advantage.69–71 Notably, a phase III trial by the Head and Neck Intergroup in the United States compared radiotherapy alone with radiotherapy plus concurrent and adjuvant chemotherapy for stage III and IV disease.69 Chemotherapy consisted of CDDP 100 mg/m2 every 3 weeks during radiotherapy and CDDP 80 mg/m2 plus 5-fluorouracil (5-FU) infusion 1000 mg/m2/day, for 4 days every 4 weeks after the completion of radiotherapy. Radiotherapy was delivered at 1.8 to 2 Gy per fraction per day, 5 days a week, to a total dose of 70 Gy. An update of the Intergroup trial72 showed that at 5 years, the overall survival was 37% versus 67% (P < 0.001) and progression-free survival was 29% versus 58% in favor of the combined modality arm (P < 0.001). Significant differences were found in the overall survival and progression-free survival between the histologic types for all patients treated. The overall survival was 37% for WHO I, 55% for WHO II, and 60% for WHO III (P < 0.001). A smaller subsequent trial suggested that even in patients with advanced local disease but minimal nodal disease (T3-4N0-1), local control increased with the use of combined chemotherapy and accelerated fractionation radiation versus conventional radiation alone.73 A substantial reduction in both locoregional recurrence and metastatic disease in the combined treatment arms versus radiation alone has been observed in these combined modality trials.
A common theme to all of these chemotherapy regimens is that in all cases the cumulative dose of concurrent CDDP exceeded 180 mg/m2. However, in the U.S. Intergroup trial, although three cycles of 100 mg/m2 of CDDP during radiotherapy were intended, only 63% of the patients received all three cycles. In the trial by Chan et al. in which CDDP was administered weekly to an anticipated total of 240 mg/m295% of the patients were compliant with the plan.74 It appears that weekly dosing is more tolerable and in many cases dosing beyond a cumulative dose of 200-240 mg/m2 is not feasible. In addition, of the three randomized controlled trials discussed, only one, the U.S. Intergroup study, administered adjuvant CDDP and 5-FU. Only approximately half of the patients were able to receive all three cycles, and one-third of the patients randomized to that arm received no adjuvant treatment. Therefore, because all three randomized trials showed an overall survival advantage for patients with advanced disease, it is not clear that adjuvant CDDP and 5-FU has benefit beyond concurrent chemoradiation, nor is it clear that administration of CDDP and 5-FU after chemoradiation is feasible.
A recent study directly compared CDDP versus carboplatin (AUC 5) in the curative setting in 206 patients with NPC who were receiving concurrent radiation.75 In this study, the standard U.S. Intergroup regimen of concurrent plus adjuvant chemoradiation was compared with an identical radiation plan with concurrent AUC 5 100 mg/m2 weekly instead of CDDP, and AUC 5 instead of CDDP in the adjuvant setting.75 There was no difference in overall survival or disease-free survival. Toxicity was markedly less in the AUC 5 arm, and more than twice as many patients in the AUC 5 arm (62% versus 26%) completed all intended chemotherapy treatment. Therefore, although CDDP is still considered the standard, substitution of AUC 5 in patients who are ineligible for CDDP because of renal or neurologic compromise is an alternative treatment approach and warrants further study.
Chemotherapy has been used as induction or neoadjuvant therapy before radiotherapy or as an adjuvant after radiotherapy in the treatment of advanced NPC.76–80 Thus far, prospective randomized trials comparing neoadjuvant or adjuvant chemotherapy combined with radiotherapy to radiotherapy alone have not shown a survival benefit, despite diminution of metastatic relapse rates.81–83 In addition, several meta-analyses did not show a benefit of neoadjuvant chemotherapy in the treatment of NPC.84–86
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree